
9H-Xanthen-9-one, 3,6-dichloro- synthesis
- Product Name:9H-Xanthen-9-one, 3,6-dichloro-
- CAS Number:1556-63-4
- Molecular formula:C13H6Cl2O2
- Molecular Weight:265.0916
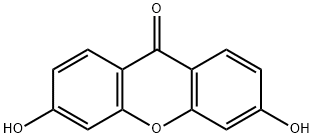
1214-24-0
65 suppliers
$25.00/100mg

1556-63-4
1 suppliers
inquiry
Yield:1556-63-4 81%
Reaction Conditions:
with thionyl chloride for 48 h;Reflux;
Steps:
4.2. Synthesis of 3,6-dichloro-9H-xanthen-9-one (32)
3,6-Dihydroxy-9H-xanthen-9-one (14, 0.200 g, 876 μmol) was dissolved in thionyl chloride (15 ml, 0.21 mol) and the reaction mixture was refluxed for 48 h. After cooling to room temperature, the reaction was carefully quenched with water. The organic phase was extracted with CH2Cl2, washed with saturated Na2CO3 (aq.) and water to give the crude product which was then purified by column chromatography using a mixture of EtOAc/Hexane (3:7) as mobile phase. 3,6-Dichloro-9H-xanthen-9-one (32); White solid (0.188 g, 81% yield); m.p.: 165-168 C; 1H NMR (Lit.43) (300 MHz, CDCl3, δ ppm): 8.25 (2H, d, 3J1,2 = 3J8,7 = 8.6 Hz, H-1, H-8), 7.50 (2H, d, 4J4,2 = 4J5,7 =1.9 Hz, H-4, H-5), 7.36 (2H, dd, 3J2,1 = 3J7,8 = 8.6 Hz and 4J2,4 = 4J7,5 =1.9 Hz, H-2, H-7) ppm.
References:
Rosa;Palmeira;Resende;Almeida;Kane-Pagès;Barreto;Sousa;Pinto [Bioorganic and Medicinal Chemistry,2021,vol. 29,art. no. 115873]
![Spiro[benzofuran-3(2H),9'-[9H]xanthen]-2-one, 3',6,6'-trichloro-](/CAS/20210305/GIF/112107-57-0.gif)
112107-57-0
0 suppliers
inquiry

1556-63-4
1 suppliers
inquiry
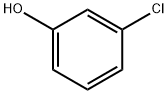
108-43-0
304 suppliers
$13.00/5g

1556-63-4
1 suppliers
inquiry
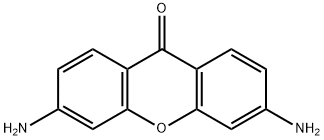
71641-67-3
5 suppliers
inquiry

1556-63-4
1 suppliers
inquiry