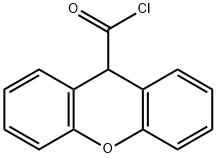
9H-XANTHENE-9-CARBONYL CHLORIDE synthesis
- Product Name:9H-XANTHENE-9-CARBONYL CHLORIDE
- CAS Number:26454-53-5
- Molecular formula:C14H9ClO2
- Molecular Weight:244.67
82-07-5
190 suppliers
$15.00/1g

26454-53-5
25 suppliers
$600.00/5G
Yield:-
Reaction Conditions:
with oxalyl dichloride;N,N-dimethyl-formamide in chloroform at 0 - 20; for 1 h;
Steps:
d
2 g of 9H-Xanthene-9-carboxylic acid (0.0088 mol) were dissolved in 30 ml of CHCI3 (ethanol free). The solution was cooled at 0°C and 1.08 ml (0.0123 mol) of oxalyl chloride and a drop of DMF was added. The mixture was stirred and allowed to warm to room temperature. After an hour at this temperature the solvents were evaporated and the residue was dissolved in CHCI3 and evaporated again. This procedure was repeated two times. The solid obtained (2.19 g) was dissolved in 20 ml of CHCI3 and added to a solution of 0.975 g (0.0097 mol) of (3R)-1-methylpyrrolidin-3-ol (Intermediate 1-19) in 15 ml of CHCI3 cooled at 0-5°C. The reaction mixture was allowed-to warm to room temperature and stirred overnight. The solvent was evaporated and the residue was dissolved in toluene and extracted with HCI 2N. The aqueous layer was basified with K2CO3 and extracted with CHCI3. The organic layer was washed with brine, dried over Na2SO4 and evaporated to dryness to yield 2.53 g (93%) of the title product as an oil. 1H-NMR (CDCl3): No. 1. 65-1.85 (m, 1 H), 2.05-2. 42 (m, 2H), 2.30 (s, 3H), 2.45-2. 60 (m, 1H), 2.60-2. 80 (m, 2H), 5.0 (s, 1 H), 5.05-5. 20 (m, 1 H), 7.0-7. 25 (m, 4H), 7.25-7. 40 (m, 4H). MS [M+1] + : 310
References:
WO2003/87094,2003,A2 Location in patent:Page/Page column 28