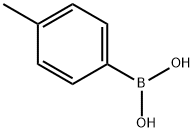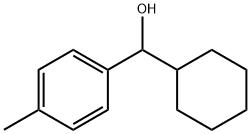
A-CYCLOHEXYL-4-METHYL-BENZENEMETHANOL synthesis
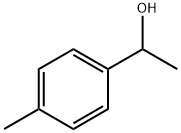
- Product Name:A-CYCLOHEXYL-4-METHYL-BENZENEMETHANOL
- CAS Number:28047-28-1
- Molecular formula:C14H20O
- Molecular Weight:204.31
Yield:28047-28-1 84%
Reaction Conditions:
with di-μ-chlorobis[(1,2,5,6-η)-1,5-cyclooctadiene]diiridium;tris(2,4-di-tert-butylphenyl)phosphite;potassium carbonate in 1,4-dioxane at 60;Glovebox;Inert atmosphere;
Steps:
General procedure for Iridium (I)-catalyzed addition reactions of arylboronic acids with aldehydes:
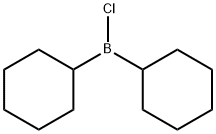
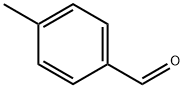
General procedure: In a glove-box with N2 atmosphere, [Ir(COD)Cl]2 (0.00125 mmol), tris(2,4-di-t-butylphenyl)phosphite 1 (0.0025 mmol) and 1,4-dioxane (0.2 mL) were placed in a vial. Then the vial containing the mixture was sealed and taken out of glove-box and heated in 110 oC of oil bath with stirring for 30 minutes. When the preparation of catalyst was completed, the vial was cooled to room temperature and brought into the glove-box again and followed by the addition of 1,4-dioxine (0.8 mL), aldehyde (0.25 mmol), arylboronic acids (0.375 mmol), and K2CO3 (0.75 mmol). After the mixture was stirred at 60 oC for 6-10 hours, the reaction was quenched by adding a small amount of water. Extraction with ethyl acetate followed by column chromatography on silica gel with ethyl acetate/hexane (v/v=1:10) afforded the alcohols.
References:
Liao, Yuan-Xi;Dong, Jie;Hu, Qiao-Sheng [Tetrahedron Letters,2018,vol. 59,# 16,p. 1548 - 1550] Location in patent:supporting information