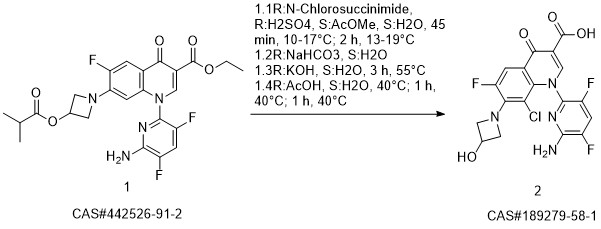
ABT-492 synthesis
- Product Name:ABT-492
- CAS Number:189279-58-1
- Molecular formula:C18H12ClF3N4O4
- Molecular Weight:440.76

Reference: Roger Hanselmann, Graham Johnson, Maxwell M. Reeve, and Shu-Ting Huang. Identification and Suppression of a Dimer Impurity in the Development of Delafloxacin. Organic Process Research & Development 2009 13 (1), 54-59. DOI: 10.1021/op800238q
![3-Quinolinecarboxylic acid, 1-(6-amino-3,5-difluoro-2-pyridinyl)-8-chloro-6-fluoro-1,4-dihydro-7-[3-(2-methyl-1-oxopropoxy)-1-azetidinyl]-4-oxo-, ethyl ester](/CAS/GIF/875712-90-6.gif)
875712-90-6
2 suppliers
inquiry

189279-58-1
147 suppliers
$25.00/1mg
Yield:189279-58-1 94%
Reaction Conditions:
with isopropyl alcohol;sodium hydroxide
Steps:
5 Preparation of delafloxacin
500 g of the compound 1 of Formula 1 prepared in Example 2 was added to a 20 L reaction flask,Adding isopropyl alcohol 10L,Stirring dissolved,Slowly drop the mass fraction of 4% sodium hydroxide solution 4L,After stirring to hydrolysis, add 5 L of acetic acid solution with a mass fraction of 12%.Fully crystallized after the filter out of solid,Water washing,Dried under reduced pressure to give pale yellow powder 384 g,Yield 94%, purity 99.3%.
References:
CHONGQING PHARMACEUTICAL RESEARCH INSTITUTE CO., LTD.;PAN, YUAN;ZUO, XIAOYONG;ZHANG, SHANGHUA;ZHANG, XIAOCHENG;FU, TINGYIN;LI, HUI;SHI, JUNPENG CN106256824, 2016, A Location in patent:Paragraph 0030

101799-75-1
5 suppliers
inquiry

189279-58-1
147 suppliers
$25.00/1mg
98349-24-7
217 suppliers
$6.00/250mg

189279-58-1
147 suppliers
$25.00/1mg

189279-51-4
10 suppliers
inquiry

189279-58-1
147 suppliers
$25.00/1mg

906088-96-8
19 suppliers
inquiry

189279-58-1
147 suppliers
$25.00/1mg