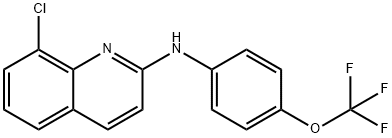
ABX-464 synthesis
- Product Name:ABX-464
- CAS Number:1258453-75-6
- Molecular formula:C16H10ClF3N2O
- Molecular Weight:338.71
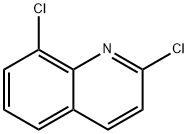
4470-83-1
122 suppliers
$11.00/250mg
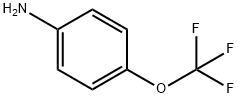
461-82-5
448 suppliers
$10.00/1g

1258453-75-6
49 suppliers
inquiry
Yield:1258453-75-6 95%
Reaction Conditions:
in isopropyl alcohol at 90;Solvent;Temperature;Concentration;
Steps:
3 3. Preparation of (8-chloro-quinolin-2-yl)-(4-trifluoromethoxyphenyl)-amine (free base) without a Palladium-Catalyst
3. Preparation of (8-chloro-quinolin-2-yl)-(4-trifluoromethoxyphenyl)-amine (free base) without a Palladium-Catalyst 2,8-Dichloroquinoline (125 g; 0.63 mol) was slurried in 4-(trifluoromethoxy)aniline (280 g; 1 .58 mol) and isopropanol (240 ml_) and the mixture was heated to 90°C. The mixture was stirred for 3-4 h when HPLC indicated complete conversion of dichloroquinoline. Thereafter, additional isopropanol (730 ml_) was added and the mixture cooled to approx. 40°C. Water (2.5 L) was added slowly and the resulting precipitate was collected by suction filtration. The filter cake was dried under reduced pressure and afterwards recrystallized from boiling cyclohexane (1 .5 L) in order to yield pure product as an off-white solid. Yield: 203 g (95%) Chemical purity: 99.9% (peak area at λ=254 nm). The identity of (8-chloro-quinolin-2-yl)-(4-trifluoromethoxyphenyl)-amine was verified by 1 H-NMR (Fig. 1 ); FT-IR (Fig. 2) and GC-MS (Fig. 3). The NMR spectrum was characterized by the following signals: 1 H NMR (400 MHz, CDCI3) δ ppm 6.87 (m, 2 H); 7.23 (m, 3 H); 7.55 (dd, J=8.01 , 1 .28 Hz, 1 H); 7.72 (dd, J=7.52, 1 .28 Hz, 1 H); 7.90 (m, 3 H). The IR-spectrum was characterized by the following signals: 3406; 1626; 1606; 1535; 1506; 1475; 1425; 1392; 1257; 1217; 1 146; 1001 ; 849; 822; 795; 754; 719; 673; 663; 631 cm"1. The solid state characteristics were investigated by means of DSC and XRPD and is as follows: The DSC thermogram (Fig. 4) is characterized by a single endotherm with an onset temperature of 120°C (± 2°C) and a peak temperature of 121 ° (± 2°C). A characteristic x-ray powder diffractogrann is given in Fig. 5 and its characteristic signals are summarized in the following table: Major peaks can be seen at angles 7.3, 14.6 and 18.3 with relative intensities of 100.0%, 86.4% and 18.3%, respectively. Further prominent peaks can be seen at angles 23.0 and 24.8 with relative intensities of 18.1 % and 35.1 %, respectively. Additional prominent peaks can be seen at angles 28.3 and 29.5 with relative intensities of 13.8% and 1 1 .2%. Finally, remarkable peaks can be seen at angles 18.6, 22.3, 24.1 , 29.0 and 42.6 with relative intensities of 8.2%, 8.0%, 7.1 %, 8.6% and 7.4%, respectively.
References:
WO2017/158201,2017,A1 Location in patent:Page/Page column 14-16