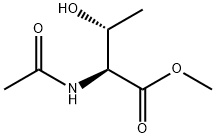
AC-THR-OME synthesis
- Product Name:AC-THR-OME
- CAS Number:2458-78-8
- Molecular formula:C7H13NO4
- Molecular Weight:175.18

39994-75-7
283 suppliers
$6.00/1g

75-36-5
561 suppliers
$17.92/100G

2458-78-8
40 suppliers
$50.00/250MG
Yield:2458-78-8 92%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in dichloromethane at 13;Cooling with ice;
Steps:
3.3.1
In a 500 mL reaction flask, IM6-1 (16.672 g, 98 mmol), 200 mL of DCM, and DIPEA (35 mL, 202 mmol) were sequentially added.Stir to dissolve, cool in ice bath,CH3COCl (7.2 mL, 101 mmol) was added dropwise,The reaction was stirred at 13 ° C, and the reaction progress was monitored by TLC-ninhydrin colorimetry.At the end of the reaction for 7.3h, suction filtration was performed under reduced pressure, and the filter cake was washed with DCM (5mL × 3).Collect the washings and filtrate, evaporate to dryness under reduced pressure (yellow oily liquid), stir with 70mL of EtOAc, suction filter under reduced pressure, wash the filter cake with EtOAc (5mL × 3),Collect the washing liquid and filtrate, evaporate to dryness under reduced pressure (yellow oily liquid),Purification by batch column chromatography (CH3OH: DCM = 1: 9 (volume ratio)),The eluate was collected, evaporated to dryness under reduced pressure, and the purity was checked by TLC-ninhydrin colorimetric method.Vacuum drying to obtain a white powdery solid IM6-215.894g, yield 92%,
References:
CN110498806,2019,A Location in patent:Paragraph 0113; 0115; 0120
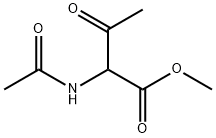
98432-01-0
1 suppliers
inquiry
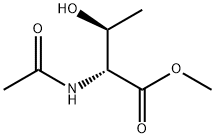
68168-94-5
0 suppliers
inquiry
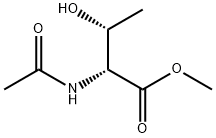
124044-17-3
0 suppliers
inquiry

2458-78-8
40 suppliers
$50.00/250MG
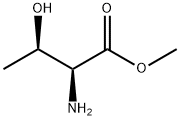
3373-59-9
58 suppliers
$44.00/5g

108-24-7
5 suppliers
$14.00/250ML

2458-78-8
40 suppliers
$50.00/250MG

73708-95-9
5 suppliers
inquiry

2458-78-8
40 suppliers
$50.00/250MG