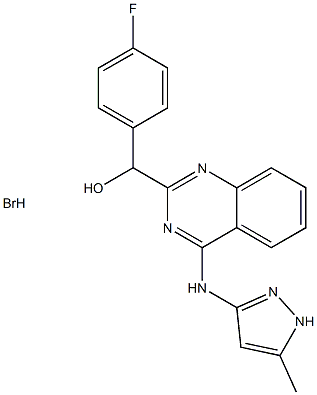
AC430 synthesis
- Product Name:AC430
- CAS Number:1359828-49-1
- Molecular formula:C19H17BrFN5O
- Molecular Weight:430.2736
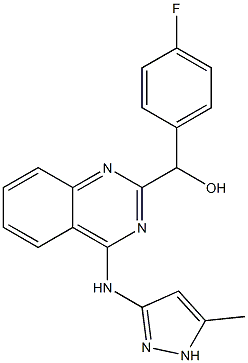
1241914-87-3
5 suppliers
inquiry

1359828-49-1
12 suppliers
inquiry
Yield: 86%
Reaction Conditions:
with hydrogen bromide in ethanol;waterReflux;
Steps:
1 Preparation of hydrobromide salts of 4-fluorophenyl)(4-(5-methyl-1H-pyrazol-3-ylamino)quinazolin-2-yl)methanol
Example 1 Preparation of hydrobromide salts of 4-fluorophenyl)(4-(5-methyl-1H-pyrazol-3-ylamino)quinazolin-2-yl)methanol A mixture of (4-fluorophenyl)(4-(5-methyl-1H-pyrazol-3-ylamino)quinazolin-2-yl)methanol (260 g, 0.744 mol) and ethanol (2.86 L) in a round bottom flask under nitrogen was heated at reflux for 30 to 40 min, and then 48% aq. HBr (125.5 g, 0.744 mmol) was added while maintaining reflux. The mixture was allowed to cool to 25-30° C., and the mixture was stirred at 25-30° C. for 1 hr. The solid was collected by filtration, washing thoroughly with fresh ethanol (0.52 L). The solid was dried at 55 to 65° C. for 12 to 14 hrs to afford (4-fluorophenyl)(4-(5-methyl-1H-pyrazol-3-ylamino)quinazolin-2-yl)methanol hydrobromide (276 g, 86%). HPLC (AUC) 99.9%.
References:
AMBIT BIOSCIENCES CORP. US2012/53195, 2012, A1 Location in patent:Page/Page column 20