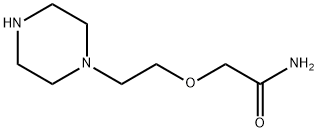
Acetamide, 2-[2-(1-piperazinyl)ethoxy]- (9CI) synthesis
- Product Name:Acetamide, 2-[2-(1-piperazinyl)ethoxy]- (9CI)
- CAS Number:197968-56-2
- Molecular formula:C8H17N3O2
- Molecular Weight:187.24
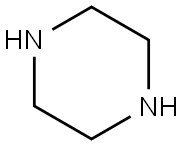
110-85-0
549 suppliers
$5.00/5G
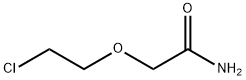
36961-64-5
154 suppliers
$18.00/1g
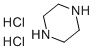
142-64-3
182 suppliers
$11.00/25g
![Acetamide, 2-[2-(1-piperazinyl)ethoxy]- (9CI)](/CAS/GIF/197968-56-2.gif)
197968-56-2
2 suppliers
inquiry
Yield:197968-56-2 57.1%
Reaction Conditions:
with ammonium hydroxide in methanol;ethanol;dichloromethane;water;butanone;
Steps:
I.1.1.2 I.1.1.2.
I.1.1.2. (variant) 8.6 g (0.1 mol) of piperazine, 15.9 g (0.1 mol) of piperazine dihydrochloride, 10.8 ml (0.1 mol) of water and 86 ml of methyl ethyl ketone are introduced into a 250 ml three-necked round-bottomed flask fitted with a water-cooled condenser and a mechanical stirrer. The mixture is brought to a temperature of 65° C. 13.8 g (0.1 mol) of (2-chloroethoxy)acetamide are than added in a single portion. The mixture is maintained at the temperature of 65° C. for 16 hours. The mixture is allowed to cool to room temperature, and the two phases are then left to separate out by settling before separating them. The lower phase (oily phase immiscible with the methyl ethyl ketone) is rinsed with 2*25 ml of methyl ethyl ketone. This oil is taken up in 50 ml of ethanol and is left stirring for 15 minutes. The precipitate formed (piperazine dihydrochloride) is filtered off and the filtrate is concentrated under reduced pressure at 50° C. on a rotary evaporator. 27 g of a yellow oil are obtained which product is purified by preparative chromatography on silica gel (eluent: 82/15/1/2 (v/v/v/v) mixture of dichloromethane/methanol/28% (weight) aqueous ammonia solution/water). 10.7 g of [2-(1-piperazinyl)ethoxy]acetamide are finally obtained in the form of a colorless oil which crystallizes. Yield: 57.1% NMR: δ: 2.33 (4H, m); 2.43 (2H, t, 5.54 Hz); 2.67 (4H, m); 2.79 (1H, bs); 3.53 (2H, t, 5.53 Hz), 3.78 (2H, s); 7.18 (1H, bs); 7.53 (1H, bs). Mass spectrum: 188 (MH+)
References:
US6140501,2000,A