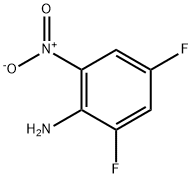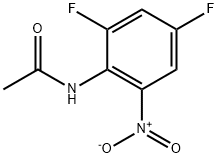
Acetamide, N-(2,4-difluoro-6-nitrophenyl)- synthesis
- Product Name:Acetamide, N-(2,4-difluoro-6-nitrophenyl)-
- CAS Number:441-30-5
- Molecular formula:C8H6F2N2O3
- Molecular Weight:216.14
Yield:441-30-5 96%
Reaction Conditions:
with pyridine in tetrahydrofuran at 18 - 25;
Steps:
Preparation of starting material Ij N-(2,4-Difluoro-6-nitro-phenyl)-acetamide:2,4-Difluoro-6-nitro-phenylamine (3.0 g, 17.1 mmol) was dissolved in anhydrous THF, to this was added pyridine (2.6 rnL, 32.4 mmol) followed by acetyl chloride (2.63 mL, 37.0 mmol). This was stirred overnight at rt under a current of N2(g). Next day TLC indicated completion of the reaction. The reaction mixture was concentrated, diluted with EtOAc, and washed with water/HCl(aq)/H2O/brine, dried under Na2SO4 and concentrated. The residue was subjected to flash chromatography on silica gel. Yield: 3.9 g (96%). 1H NMR (300 MHz, CDCl3) δ: 8.0 (brs, IH), 7.5-7.6 (m, IH), 7.23-7.59 (m, IH), 2.28 (s, 3H). (M+l)/Z = 217.1.
References:
WO2008/56150,2008,A1 Location in patent:Page/Page column 181
399-36-0
84 suppliers
$20.00/5g

441-30-5
5 suppliers
inquiry