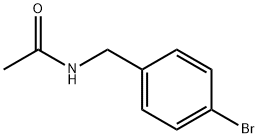
AcetaMide, N-[(4-broMophenyl)Methyl]- synthesis
- Product Name:AcetaMide, N-[(4-broMophenyl)Methyl]-
- CAS Number:90561-76-5
- Molecular formula:C9H10BrNO
- Molecular Weight:228.09
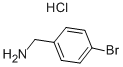
26177-44-6
181 suppliers
$6.00/1g

75-36-5
590 suppliers
$17.92/100G
![AcetaMide, N-[(4-broMophenyl)Methyl]-](/CAS/GIF/90561-76-5.gif)
90561-76-5
29 suppliers
$45.00/25mg
Yield:90561-76-5 100%
Reaction Conditions:
with triethylamine in dichloromethane at 0 - 30; for 2 h;
Steps:
48.48a
Step 48a: N-(4-bromobenzyl)acetamide (Compound 0601-135)To the solution of 4-bromobenzylamine hydrochloride (1.2 g, 5.4 mmol) and Et3N (5.5 g, 54 mmol) in dichloromethane (10 mL) was added CH3COCl (555 mg, 7.02 mmol) at 0 °C and stirred for 2 hr at 30 °C. Then the mixture was concentrated and the residue was dissolved in CH2C12 (30 mL), washed with water, dried over Na2S04 and concentrated to obtain 0601-135 (1.3 g, 100%) as a yellow solid. LCMS: 228 [M+l]+, 1H NMR (400 MHz, OMSO-d6) δ 2.00 (s, 3H), 4.33 (d, J= 6.4 Hz, 2H), 6.26 (s, 1H), 7.13 (d, J= 8.4 Hz, 2H), 7.43 (d, J= 8.4 Hz, 2H).
References:
WO2011/130628,2011,A1 Location in patent:Page/Page column 183-184
106-38-7
412 suppliers
$14.00/5g
75-05-8
1013 suppliers
$10.00/10g
![AcetaMide, N-[(4-broMophenyl)Methyl]-](/CAS/GIF/90561-76-5.gif)
90561-76-5
29 suppliers
$45.00/25mg
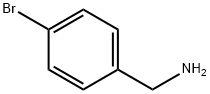
3959-07-7
199 suppliers
$10.00/1g

108-24-7
0 suppliers
$14.00/250ML
![AcetaMide, N-[(4-broMophenyl)Methyl]-](/CAS/GIF/90561-76-5.gif)
90561-76-5
29 suppliers
$45.00/25mg

3959-07-7
199 suppliers
$10.00/1g
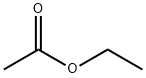
141-78-6
655 suppliers
$22.37/250ml
![AcetaMide, N-[(4-broMophenyl)Methyl]-](/CAS/GIF/90561-76-5.gif)
90561-76-5
29 suppliers
$45.00/25mg
201230-82-2
1 suppliers
inquiry
80-43-3
0 suppliers
$36.70/100g

3959-07-7
199 suppliers
$10.00/1g
![AcetaMide, N-[(4-broMophenyl)Methyl]-](/CAS/GIF/90561-76-5.gif)
90561-76-5
29 suppliers
$45.00/25mg