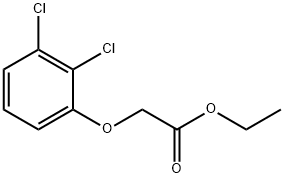
Acetic acid, (2,3-dichlorophenoxy)-, ethyl ester synthesis
- Product Name:Acetic acid, (2,3-dichlorophenoxy)-, ethyl ester
- CAS Number:37536-92-8
- Molecular formula:C10H10Cl2O3
- Molecular Weight:249.09

623-50-7
256 suppliers
$5.00/1g
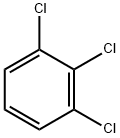
87-61-6
214 suppliers
$14.00/25g

37536-92-8
11 suppliers
$106.00/250mg
Yield:37536-92-8 99.1%
Reaction Conditions:
Stage #1: ethyl 2-hydroxyacetatewith potassium methanolate at 30;
Stage #2: 1,2,3-trichlorobenzene at 100;
Steps:
7
To the obtained ethyl hydroxyacetate, 77.53 g of 99.5% potassium methoxide was added.The reaction-formed alcohol was simultaneously removed at 30 ° C, and 216.26 g of 99% 1,2,3-trichlorobenzene was added thereto, and the condensation reaction was carried out at 100 ° C.After the reaction, the unreacted 1,2,3-trichlorobenzene was removed under reduced pressure.The condensation liquid is cooled to 40 ° C for filtration, and the filter cake is dried under reduced pressure.The dried fractions are collected and combined with the filtrate.GotEthyl 2,3-dichlorophenoxyacetate250.88g, content 98.4%,The yield was 99.1%.
References:
CN108947816,2018,A Location in patent:Paragraph 0064; 0066
576-24-9
239 suppliers
$14.00/1g

105-36-2
345 suppliers
$5.00/5G

37536-92-8
11 suppliers
$106.00/250mg