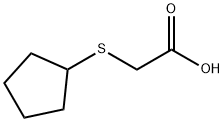
Acetic acid, (cyclopentylthio)- (9CI) synthesis
- Product Name:Acetic acid, (cyclopentylthio)- (9CI)
- CAS Number:52363-14-1
- Molecular formula:C7H12O2S
- Molecular Weight:160.23
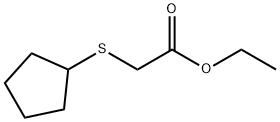
60811-66-7
0 suppliers
inquiry

52363-14-1
12 suppliers
$135.00/50mg
Yield:-
Reaction Conditions:
Stage #1: ethyl 2-(cyclopentylthio)acetatewith water;potassium hydroxide in ethanol; for 3 h;Reflux;
Stage #2: with hydrogenchloride in dichloromethane;water; pH=~ 2;Cooling with ice;
Steps:
143.143B
Example 143B 2-(cyclopentylthio)acetic acid A solution of 20% aqueous KOH (14.8 g, 52.8 mmol) was added to a solution of the product from Example 143A (8.89 g, 46.7 mmol) in EtOH (30 mL), and the resulting mixture was heated at reflux for 3 hours. The reaction solution was cooled to room temperature and concentrated under vacuum. The residue was diluted with water (20 mL) and CH2Cl2 (50 mL) and cooled in ice as 37% HCl (15 mL) was added gradually with good stirring (final pH ~2). The aqueous layer was separated and extracted with CH2Cl2 (50 mL) and the combined organic phase was washed with brine (30 mL), dried (Na2SO4), filtered, and concentrated under vacuum to provide the title compound. 1H NMR (300 MHz, CDCl3) δ ppm 1.44-1.66 (m, 4H), 1.67-1.86 (m, 2H), 1.93-2.12 (m, 2H), 3.14-3.31 (m, 1H), 3.30 (s, 2H).
References:
US2012/122888,2012,A1 Location in patent:Page/Page column 44-45
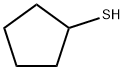
1679-07-8
125 suppliers
$12.00/1g

52363-14-1
12 suppliers
$135.00/50mg