Acetyltriacetonamin synthesis
- Product Name:Acetyltriacetonamin
- CAS Number:52326-65-5
- Molecular formula:
- Molecular Weight:0
Yield:52326-65-5 88%
Reaction Conditions:
at 100; for 7 h;
Steps:
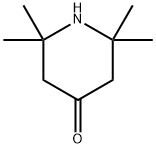
2.7 N-Acetyl-2,2,6,6-tetramethyl-4-piperidone (8)
A mixture of 2,2,6,6-tetramethyl-4-piperidone (4.91 g, 31.6 mmol) and 20 mL of acetic anhydride was heated at 100 °C for 7 h.
Solvent was removed, 10% NaOH aqueous solution was added, and the mixture was stirred.
The mixture was extracted with CH2Cl2, washed with brine, dried over Na2SO4, and then concentrated.
The residue was purified by column chromatography on silica gel with 3:1 n-hexane/AcOEt to afford the title compound 8 (5.49 g, 88%) as a colorless liquid. 1H NMR (395 MHz, CDCl3) δ (ppm) 2.59 (s, 4H), 2.23 (s, 3H), 1.54 (s, 12H); MS (EI) m/z 197 (M+), 140 (100%).
References:
Sato, Manabu;Ohta, Kiminori;Kaise, Asako;Aoto, Sayaka;Endo, Yasuyuki [Bioorganic and Medicinal Chemistry,2016,vol. 24,# 5,p. 1089 - 1094]
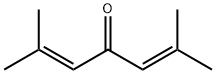
504-20-1
162 suppliers
$10.00/250mg

52326-65-5
8 suppliers
inquiry

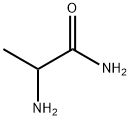
![Propanamide, 2-[(1-acetyl-2,2,6,6-tetramethyl-4-piperidinylidene)amino]-](/CAS/20210305/GIF/142080-51-1.gif)