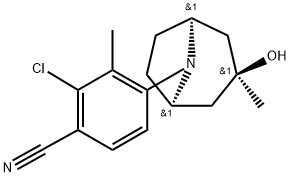
ACP-105 synthesis
- Product Name:ACP-105
- CAS Number:899821-23-9
- Molecular formula:C16H19ClN2O
- Molecular Weight:290.79
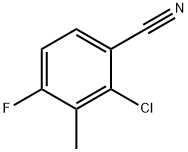
796600-15-2
132 suppliers
$10.00/250mg
![3-methyl-3-hydroxy-8-azabicyclo[3.2.1]octane hydrochloride](/CAS/GIF/870889-89-7.gif)
870889-89-7
22 suppliers
$460.00/1g

899821-23-9
113 suppliers
inquiry
Yield:899821-23-9 40%
Reaction Conditions:
with potassium carbonate in dimethyl sulfoxide at 80; for 18 h;
Steps:
26
Example 26 2-Chloro-4-(3-endo-hydroxy-3-exo-methyl-8-azabicyclo[3.2.1]oct-8-yl)-3-methylbenzonitrile (198RL93) 2-Chloro-4-fluoro-3-methylbenzonitrile (198RL18, 2.48 g, 14.6 mmol), endo-3-exo-methyl-8-azabicyclo[3.2.1]octan-3-ol hydrochloride (197FBA20a, 3.37 g, 19.0 mmol), and potassium carbonate (6.67 g, 48.2 mmol) were dissolved in dimethyl sulphoxide (40 mL), and the mixture stirred under argon at 80° C. for 18 hours. The reaction mixture was poured into water (200 mL) and stirred for 30 min. The off-white solid was filtered off and recrystallised twice from toluene, giving a white powder (1.53 g). The mother liquor was evaporated and the residue recrystallised to yield a second batch of compound (210 mg), giving an overall yield of 40%. Mp=145-147° C. Rf=0.68 (ethyl acetate/dichloromethane 1:1) LC/MS m/z 291 [M+H]+. 1H-NMR (CDCl3) δ 7.39 (d, 1H, J=8.6, Ar-H), 6.84 (d, 1H, J=8.6, Ar-H), 3.82 (m, 2H, Tr-H), 2.36 (s, 3H, Ar-CH3), 2.32-2.22 (m, 2H, Tr-H), 2.17-1.98 (m, 2H, Tr-H), 1.92-1.77 (m, 4H, Tr-H), 1.26 (s, 3H, Tr-CH3).
References:
US2006/160845,2006,A1 Location in patent:Page/Page column 22