ACVJLORANYOXLT-LZYBPNLTSA-N synthesis
- Product Name:ACVJLORANYOXLT-LZYBPNLTSA-N
- CAS Number:57755-34-7
- Molecular formula:C14H15N3O2
- Molecular Weight:257.2878
Yield:57755-34-7 83%
Reaction Conditions:
in ethanol at 20;
Steps:
2.2. General Procedure for the Synthesis of 1a-g and 2a-f
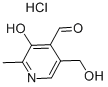
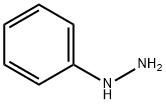
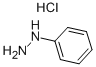
General procedure: The desired compounds 1a-g and 2a-f were prepared by reaction between pyridoxal hydrochloride (0.15 g, 0.74mmol) and the appropriate aromatic or heteroaromatic hydrazine or N-acylhydrazine (1.1 eq., 0.81mmol) in ethanol (10.0 mL). The reaction mixture was stirred for 1-48 hours at room temperature. After that, product was purified by wash-ing with cold ethanol (3.0 mL) and cold diethyl ether (3.0 mL), leading to the pure derivatives 1a-g and 2a-f as solid in 42-86% yields.
References:
Nogueira, Thais Cristina Mendon?a;Cruz, Lucas Dos Santos;Louren?o, Maria Cristina;de Souza, Marcus Vinicius Nora [Letters in drug design and discovery,2019,vol. 16,# 7,p. 792 - 798]