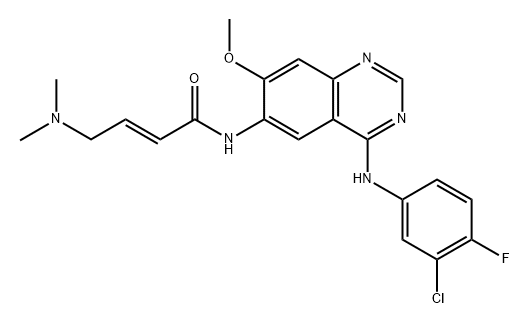
Afatinib Impurity 19 synthesis
- Product Name:Afatinib Impurity 19
- CAS Number:1245555-42-3
- Molecular formula:C21H21ClFN5O2
- Molecular Weight:429.88
![2-Butenamide, 4-bromo-N-[4-[(3-chloro-4-fluorophenyl)amino]-7-methoxy-6-quinazolinyl]-, (2E)-](/CAS/20200515/GIF/1245555-80-9.gif)
1245555-80-9
0 suppliers
inquiry

124-40-3
509 suppliers
$18.00/100ml

1245555-42-3
11 suppliers
inquiry
Yield:-
Reaction Conditions:
in ISOPROPYLAMIDE;water at 0;
Steps:
A.1.1.1
To a stirred solution of the above crude (2E)-4-brorno-N-[4-(3-chloro-4-fluoroanilino)-7-methoxy- 6-quinazolinyl]-2-butenamide (1.0 g, 2.15 mmol) in dimethylacetamide (35 mL) at 0 °C was added excess aqueous dimethylamine solution (40 %, 5 mL). After 3 hours the reaction was diluted with brine and extracted with ethyl acetate (3x). The combined organic extracts were washed with brine, dried over anhydrous Na2SO4 and concentrated under reduced pressure. Chromatography on silica gel elutingwith dichloromethane/methanol (5:95 to 15:85) then gave (2jE)-N-[4-(3-chloro-4- fluoroanilino)-7-methoxy-6-quinazolmyl]-4-(dirned3ylamino)-2-butenaniide (14) (0.74 g, 80%) as a white solid, m.p. (MeOH) 179-181°C. 1H NMR [(CDj)2SO] δ 9.78 (s, 1 H), 9.64 (s, 1 H), 8.93 (s, 1 H), 8.53 (s, 1 H), 8.13 (ddj = 2.6, 6.9 Hz, 1 H), 7.83-7.79 (m, 1 H), 7.42 (ddj = 9.1 Hz, JHF = 9.1 Hz, 1 H), 7.29 (s, 1 H), 6.80 (pi, J = 6.0, 15.4 Hz, 1 H), 6.58 (dj = 15.4 Hz, 1 H), 4.01 (s, 3 H), 3.08 (d J = 6.0 Hz, 2 H), 2.19 (s, 6 H). Analysis found: C, 56.32; H, 5.14; N, 15.73. C21H21ClFN5O2-H2O requires: C, 56.32; H, 5.18; N, 15.64.
References:
WO2010/104406,2010,A1 Location in patent:Page/Page column 34; 43-44