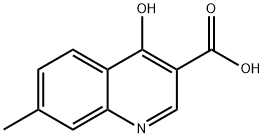
AKOS BB-8877 synthesis
- Product Name:AKOS BB-8877
- CAS Number:256923-25-8
- Molecular formula:C11H9NO3
- Molecular Weight:203.19
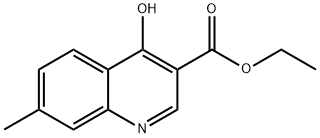
41460-18-8
6 suppliers
inquiry

256923-25-8
5 suppliers
inquiry
Yield:256923-25-8 92%
Reaction Conditions:
with sodium hydroxide in lithium hydroxide monohydrate; for 0.5 h;Reflux;
Steps:
4-Hydroxy-7-methylquinoline-3-carboxylicacid (5a)
4a (4.31 g, 18.65 mmol) was then hydrolyzed by refluxing in 2M sodium hydroxide (80 mL) and monitoring completion of the reaction by solubilization (~30 minutes). Upon cooling to room temperature, pH of the mixture was adjusted to about 4 with 2M HCl. The resultant solid was filtered, washed with water and dried. 5a (3.50 g, 92%) was obtained as a white solid, mp 254-255 °C. δH (400 MHz; CDCl3) 2.52 (3H, s, CH3), 6.83 (1H, dd, J 2.4, 9.0 Hz, H-6), 7.29 (1H, d, J 1.7 H-8), 7.89 (1H, d, J 9.1 Hz, H-5), 8.73 (1H, s, H-2), 13.85 (1H, br s, Ar-OH). δC (100 MHz; CDCl3) 21.9 (Ar-CH3), 107.8 (C-3), 119.2 (C-4a), 122.8 (C-5), 125.3 (C-8), 128.3 (C-6), 133.5 (C-7), 140.1 (C-2), 145.0 (C-8a), 166.9 (C-4), 178.5 (Ar-COOH ).
References:
Nsumiwa, Samkele;Kuter, David;Wittlin, Sergio;Chibale, Kelly;Egan, Timothy J. [Bioorganic and Medicinal Chemistry,2013,vol. 21,# 13,p. 3738 - 3748] Location in patent:supporting information
![Propanedioic acid, 2-[[(3-methylphenyl)amino]methylene]-, 1,3-diethyl ester](/CAS/20210305/GIF/19056-83-8.gif)
19056-83-8
1 suppliers
inquiry

256923-25-8
5 suppliers
inquiry
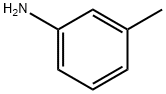
108-44-1
367 suppliers
$9.00/1g

256923-25-8
5 suppliers
inquiry