Alanine, 2-methyl-N-(phenylmethyl)-, methyl ester synthesis
- Product Name:Alanine, 2-methyl-N-(phenylmethyl)-, methyl ester
- CAS Number:78002-18-3
- Molecular formula:C12H17NO2
- Molecular Weight:207.27
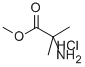
15028-41-8
198 suppliers
$9.00/10g
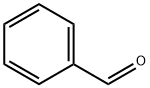
100-52-7
938 suppliers
$20.37/250g

78002-18-3
1 suppliers
inquiry
Yield:78002-18-3 98%
Reaction Conditions:
Stage #1: methyl 2-aminoisobutyrate hydrochloride;benzaldehydewith magnesium sulfate;triethylamine in tetrahydrofuran at 20; for 12 h;
Stage #2: with sodium tetrahydroborate in methanol at 0 - 20; for 1 h;
Steps:
Methyl 2-(benzylamino)-2-methylpropanoate (2):
To a solution of methyl 2-amino-2-methylpropanoate hydrochloride 1 (503 mg, 3.27 mmol) in THF (5 ml) was added MgSO4 (300 mg, 3.27 mmol), TEA (0.456 ml, 3.27 mmol), and benzaldehyde (0.33 ml, 3.27 mmol). The suspension was stirred at room temperature overnight. The reaction suspension was filtered, washed with THF, and the filtrate concentrated in vacuo. The residue obtained was taken up in MeOH (10 ml) and cooled to 0 °C. NaBH4 (149 mg, 3.93 mmol) was added in several portions over a period of 10 minutes. After the addition was complete, the reaction was warmed to room temperature and stirred for 1h. Water was added and the product was extracted with EtOAc. The organic layer was combined, washed with brine, dried over anhydrous Na2SO4, filtered, and concentrated in vacuo to afford compound 2 (620 mg, 98 %) which was used in the next step without further purification. 1H NMR (500 MHz, CDCl3) δ 1.34 - 1.41 (m, 6 H), 3.62 (br. s., 2 H), 3.74 (br. s., 3 H), 4.69 (br. s., 1 H), 7.32 - 7.39 (m, 5 H).
References:
Malik, Neha;Iyamu, Iredia D.;Scheidt, Karl A.;Schiltz, Gary E. [Tetrahedron Letters,2018,vol. 59,# 15,p. 1513 - 1516] Location in patent:supporting information
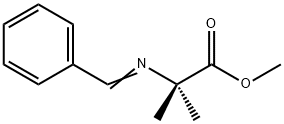
32273-76-0
0 suppliers
inquiry

78002-18-3
1 suppliers
inquiry