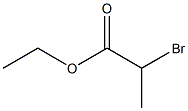
Alanine, N,N-dimethyl-, ethyl ester synthesis
- Product Name:Alanine, N,N-dimethyl-, ethyl ester
- CAS Number:82614-49-1
- Molecular formula:C7H15NO2
- Molecular Weight:145.2
Yield:82614-49-1 33%
Reaction Conditions:
Stage #1: dimethyl amine;Ethyl 2-bromopropionatewith potassium carbonate in water;acetonitrile at 20; for 17.5 h;Inert atmosphere;
Stage #2: with sodium hydroxide in water;acetonitrile;
Steps:
60
[0303] Compound 1 (Ethyl 2-bromopropionate, 1.5 g, 8.29 mmol) was dissolved in MeCN (22 ml). K2CO3 (3.5 g, 24.86mmol) was added thereto, followed by stirring under a nitrogen atmosphere. Dimethylamine solution (40 wt% in H2O)(3 ml, 24.86 mmol) was added thereto, followed by stirring for 17.5 hours at room temperature. An aqueous 1 N NaOHsolution (22 ml) was added thereto and further stirred for 10 minutes. Distilled water and EA were added to a reactionsolution, extracted several times, dried over MgSO4 and then filtered. The filtered solution was vacuum evaporated andthen intactly collected.Colorless oil 0.397 g (33%)
References:
EP3091003,2016,A1 Location in patent:Paragraph 0302; 0303

64-17-5
735 suppliers
$10.00/50g

19701-89-4
27 suppliers
$58.00/100mg

82614-49-1
0 suppliers
inquiry

124-40-3
523 suppliers
$18.00/100ml
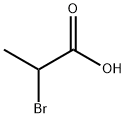
598-72-1
357 suppliers
$16.00/25g

82614-49-1
0 suppliers
inquiry