
ALLYL(DIISOPROPYL)(4-METHOXYPHENYL)SILANE synthesis
- Product Name:ALLYL(DIISOPROPYL)(4-METHOXYPHENYL)SILANE
- CAS Number:216107-40-3
- Molecular formula:C16H26OSi
- Molecular Weight:262.46
![Benzene, 1-[chlorobis(1-methylethyl)silyl]-4-methoxy-](/CAS/20210305/GIF/347904-48-7.gif)
347904-48-7
0 suppliers
inquiry
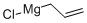
2622-05-1
153 suppliers
$40.00/500mg

216107-40-3
14 suppliers
$236.81/1gm:
Yield:216107-40-3 94%
Reaction Conditions:
in tetrahydrofuran at 0; for 3 h;
Steps:
2.II
[0387] Allyl(4-methoxyphenyl)diisopropylsilane. To the crude chloro(4methoxyphenyl)-diisopropylsilane (54.8 g, 214 mmol, 1.0 equiv.) was added THF (335 mL) via cannula under Ar. The solution was chilled to 0° C. and treated with allylmagnesium chloride (128 mL, 256 mmol, 2.0 M in THF, 1.2 equiv.). After 3 h at 0° C., the solution was allowed to warm to 23° C. with stirring overnight (16 h). The mixture was treated with saturated NH4Cl (50 mL), and the aqueous layer was extracted with ether (3×500 mL). The combined organic extracts were washed with brine, dried over MgSO4, filtered, and concentrated in vacuo. The crude material was purified by silica flash chromatography (3-5% EtOAc/hexanes) to yield 52.86 g (94%) of a slightly cloudy, clear viscous oil. This reagent distills at 80° C. at 500 mTorr as a colorless oil. TLC Rf=0.40 (1:9 EtOAc/hexanes). IR(film): 2942, 2865, 1630, 1595, 1504, 1463, 1277. 1H NMR (500 MHz, CDCl3): δ7.32 (d, 2H, J=6.84), 6.81(d, 2H, J=6.84), 5.82 (q, 1H, J=8.5, 8.5), 4.88 (d, 1H, J=17.05), 4.76 (d, 1H, J=9.77), 1.82 (d, 2H, J=7.32), 1.17 (q, 2H, J=7.3), 0.94 (d, 6H, J=7.3), 0.90 (d, 6H, J=7.3). 13C NMR (126 MHz, CDCl3): δ160.51, 136.48, 135.70, 125.78, 113.78, 113.62, 55.09, 19.34, 18.22, 18.17, 17.68, 11.30. Elemental analysis, Calcd.: C 73.22, H 9.98, Si 10.70. Found: C 73.25, H 9.97, Si 10.77.
References:
US2004/59138,2004,A1 Location in patent:Page 58-59
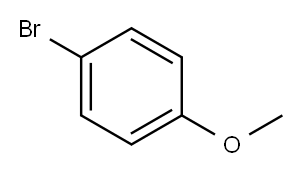
104-92-7
424 suppliers
$10.00/10g

216107-40-3
14 suppliers
$236.81/1gm:
![Benzene, 1-[bis(1-methylethyl)silyl]-4-methoxy-](/CAS/20210305/GIF/347904-47-6.gif)
347904-47-6
0 suppliers
inquiry

216107-40-3
14 suppliers
$236.81/1gm:
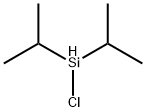
2227-29-4
114 suppliers
$40.00/2g

216107-40-3
14 suppliers
$236.81/1gm: