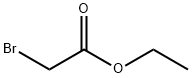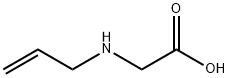
ALLYLAMINO-ACETIC ACID HYDROCHLORIDE synthesis
- Product Name:ALLYLAMINO-ACETIC ACID HYDROCHLORIDE
- CAS Number:3182-77-2
- Molecular formula:C5H9NO2
- Molecular Weight:115.13
Yield:-
Reaction Conditions:
with potassium carbonate;triethylamine;sodium iodide in N,N-dimethyl-formamide at 0 - 20;
Steps:
17
Preparation 17
2-(Allyl(tert-butoxycarbonyl)amino)acetic acid
To an Erlenmeyer flask containing potassium carbonate (100 g, 724 mmol), sodium iodide (110 g, 727 mmol), dimethylformamide (300 mL), triethylamine (200 mL, 1.44 mole) and 2-propen-1-amine (24 g, 426 mmol) at 0° C. is added drop wise a solution of ethyl 2-bromoacetate (60.2 g, 360 mmol) in dimethylformamide (40 mL).
The reaction is warmed to ambient temperature and stirred overnight.
The mixture is filtered, washed with diethyl ether (200 mL), and concentrated.
Brine (1 L) is added to the filtrate and the layers are separated.
The aqueous layer is extracted with diethyl ether (3*500 mL).
The organic phases are combined, dried over magnesium sulfate, filtered, and the solvent removed under reduced pressure to give a residue.
To a solution at 0° C. of crude residue in ethanol (500 ml) and triethylamine (40 g, 395 mmol) is added di-t-butyldicarbonate (105 g, 467 mmol) in one portion.
The reaction is warmed to room temperature and stirred overnight.
The reaction is concentrated under reduced pressure, diluted with water (200 mL) and saturated sodium bicarbonate (200 mL), and extracted with ethyl acetate (2*200 mL) and dichloromethane (200 mL).
The organic phases are combined, dried over magnesium sulfate, filtered, and concentrated under reduced pressure to give a crude residue.
The crude residue is taken up in methanol (200 mL) and 2 N sodium hydroxide (500 mL) is added and the mixture is stirred for approximately 3 hours at room temperature.
The solution is concentrated under reduced pressure and the resulting aqueous solution acidified to pH 4 with 12 N hydrochloric acid.
The resulting precipitate is collected by filtration, washed with water, and dried to give the title compound (50 g, 65%).
1H NMR (CDCl3) mixture of two rotamers (50:50) δ 1.43, 1.45 (s, 9H), 3.86-3.99 (m, 4H), 5.10-5.20 (m, 2H), 5.71-5.83 (m, 1H).
References:
US2013/261111,2013,A1 Location in patent:Paragraph 0089; 0090
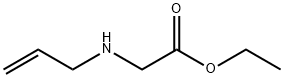
3182-79-4
12 suppliers
inquiry

3182-77-2
22 suppliers
inquiry
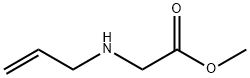
10552-80-4
2 suppliers
inquiry

3182-77-2
22 suppliers
inquiry