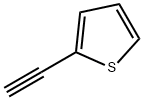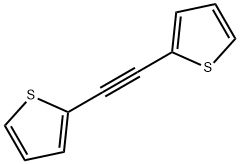
alpha,alpha'-Dithienylacetylene synthesis
- Product Name:alpha,alpha'-Dithienylacetylene
- CAS Number:23975-15-7
- Molecular formula:C10H6S2
- Molecular Weight:190.28
3437-95-4
245 suppliers
$6.00/5g

1010840-17-1
34 suppliers
$134.00/1G

23975-15-7
18 suppliers
inquiry
Yield:23975-15-7 99 %Spectr.
Reaction Conditions:
with tri-tert-butyl phosphine;2,6-di-tert-butyl-4-methyl-phenol;tetrabutylammomium bromide;palladium diacetate;diisopropylamine in dimethyl sulfoxide at 200; for 0.0833333 h;Inert atmosphere;Microwave irradiation;Suzuki-Miyaura Coupling;
Steps:
1,2-Diphenylethyne (5)
General procedure: A 5 ml of microwave vial (Biotage) was charged with, iodobenzene (16.7 mg, 81.9 mmole), B2C2 (4.3 mg, 15.5 mmole), Pd(OAc)2 (0.93 mg, 4.2 mmole), P(tBu)3 (3.5 mg, 17 mmole), tetrabutylammonium bromide (44 mg, 136 mmole), dibutylhydroxytoluene (1 mg) and a magnetic stir bar. After sealing with a vial cap, this vessel was applied to vacuum and charged with nitrogen. The vacuuming/charging cycle was repeated 3 times. DMSO/iPr2NH mixture (10/1, 0.45 ml) previously bubbled with nitrogen for 1 hour (~20 ml stock solution), was added via syringe. The vessel was placed into the microwave cavity. The reaction mixture was stirred at room temperature for 1min and then 30 W microwave was irradiated. The temperature was ramped from room temperature to the set-up point (200°C). Once the set-up temperature was reached, the reaction mixture was held at that temperature for 5 min. After the mixture was allowed to cool to room temperature by internal cooling system, the reaction vessel was opened. The thick solution was poured onto the glass filter and was washed 5 times with ether (55 mL). The organic layer was washed with brine (210 mL) and then with H2O, following which it was dried over MgSO4, filtered, and evaporated. Obtained solids were passed through a short pad of silica gel with chloroform as an eluent and solvents were evaporated under vacuum. Collected solids were dissolvedin ~1 mL of CDCl3 with 3 mg of hexamethylbenzene as an internal reference, 1H-NMR was measured to check the product peaks as well as yield. (90% based on 7.6 mg of 1,2-Bis(4’,4’,5’,5’-tetramethyl[1’,3’,2’]dioxaborolan-2’-yl)ethyne.1H-NMR peaks were consistent with those reported in the literature. Same procedure starting from bromobenzene gave 80% yield.
References:
Jung, Daero;Kang, Youn Kyung [Bulletin of the Korean Chemical Society,2016,vol. 37,# 4,p. 576 - 579] Location in patent:supporting information
1003-09-4
469 suppliers
$10.00/25g
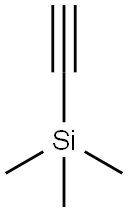
1066-54-2
470 suppliers
$9.00/10g

23975-15-7
18 suppliers
inquiry
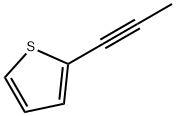
23229-66-5
0 suppliers
inquiry

23975-15-7
18 suppliers
inquiry