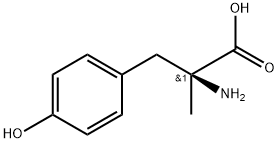
ALPHA-METHYL-L-P-TYROSINE synthesis
- Product Name:ALPHA-METHYL-L-P-TYROSINE
- CAS Number:672-87-7
- Molecular formula:C10H13NO3
- Molecular Weight:195.22

1299492-09-3
0 suppliers
inquiry

672-87-7
172 suppliers
$7.00/5mg
Yield:672-87-7 84%
Reaction Conditions:
with water;hydrogen bromide at 120; for 5 h;Product distribution / selectivity;
Steps:
6
Example 6Metyrosine 1 To a 100 mL flask with a reflux condenser was charged amide 4 (5.50 g of a mixture containing 4 (3.37 g, 16.1 mmol) and phenylacetamide (2.13 g)) and 48% HBr (30 mL). The solution was heated for 5 h at 120° C. and was cooled to room temperature. H2O (60 mL) was added and the solution was washed with EtOAc (3×35 mL). The aqueous phase was concentrated in vacuo to provide a beige paste. The paste was dissolved in H2O (15 mL) and the resulting mixture was heated to 65° C. Activated carbon (300 mg, Type NORIT SX) was added and the mixture was stirred for 15 min, was filtered and the filter pad was washed with water (2×4 mL). The combined filtrates were heated to 55° C. and the pH was adjusted to 5-6 using 32% aq. NH3. The mixture was cooled to 0° C., was stirred for 15 min and was filtered. The collected solids were washed with cold water (2×5 mL) and were dried in vacuo to provide (-)-α-methyl-L-tyrosine (or metyrosine) 1 (2.65 g, 84%) as a white solid. HPLC (Zorbax C18, NaH2PO4 10 mM pH=3/MeCN (100:0) 10 min, (100:0) to (0:100) 15 min, 0:100 5 min) tR=10.1 min. Chiral HPLC (Nucleosil Chiral-1, CuSO4 10 mM/MeCN 10:1) tR=16.9 min. m.p.=320-321° C. [α]546=+201° (c=0.5 Copper complex solution) (lit.2+185-190° Copper complex solution preparation: Solution A (anhydrous NaOAc dissolved in H2O (150 mL) in 250 mL volumetric flask, glacial acetic acid (50 mL) added and diluted to volume with H2O) mixed with Solution B (cupric sulfate (62.5 g) diluted to volume with H2O in a 200 mL volumetric flask) in a 1 L volumetric flask and was diluted to volume with H2O. Metyrosine solution (5 mg/mL) was prepared in this solution.To obtain an NMR spectrum (taking into account the low solubility of the product), a small sample (10 mg) was transformed into its HCl salt. The sample was dissolved in 2 M HCl and the solution was evaporated to dryness. 1H NMR (D2O, 400 MHz) 1.49 (s, 3H); 2.90 (d, J=14.5 Hz, 1H); 3.16 (d, J=14.5 Hz, 1H); 6.75 (d, J=8.2 Hz, 2H), 7.01 ((d, J=8.2 Hz, 2H). 13C NMR (D2O, 100.6 MHz) 21.6; 41.6; 61.0; 116.0, 125.0; 131.7; 155.5; 173.8.
References:
US2011/104765,2011,A1 Location in patent:Page/Page column 14
1299492-14-0
0 suppliers
inquiry

672-87-7
172 suppliers
$7.00/5mg
1299492-20-8
0 suppliers
inquiry

672-87-7
172 suppliers
$7.00/5mg
623-05-2
667 suppliers
$5.00/100mg

672-87-7
172 suppliers
$7.00/5mg
2746-25-0
173 suppliers
$27.57/250mgs:

672-87-7
172 suppliers
$7.00/5mg