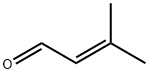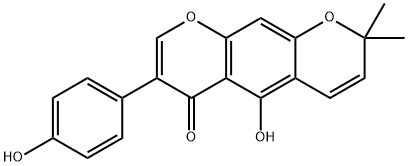
alpinumisoflavone synthesis
- Product Name:alpinumisoflavone
- CAS Number:34086-50-5
- Molecular formula:C20H16O5
- Molecular Weight:336.34
Yield: 38%
Reaction Conditions:
with calcium hydroxide in methanol at 18; for 48 h;
Steps:
1.1; 2.1 1. Preparation of intermediate 3
The genistein 2 (4.0 g, 29.6 mmol) was dissolved in 300 mL of methanol. 3-methyl-2-butenal (14.4 mL, 148.0 mmol) and calcium hydroxide (4.4 g, 59.2 mmol) were added. The reaction solution was stirred at room temperature (18 ° C) for 48 h and then concentrated. The obtained sample was dissolved in 300 mL of ethyl acetate and washed with 1 M aqueous hydrochloric acid. The organic phase was separated, dried over anhydrous sodium sulfate and filtered then filtered. The crude product was purified by silica gel column chromatography using petroleum ether and ethyl acetate. 3.8 g of intermediate 3 were obtained as a yellow solid, yield 38%.
References:
Jinan University;Bai Weibin;Lan Ping;Jiang Xinwei;Sun Jianxia;Tian Lingmin CN110283182, 2019, A Location in patent:Paragraph 0035; 0037; 0039-0040; 0051; 0053; 0055
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

34086-50-5
71 suppliers
inquiry

124360-59-4
2 suppliers
inquiry

34086-50-5
71 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

34086-50-5
71 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

34086-50-5
71 suppliers
inquiry