anitrazafen synthesis
- Product Name:anitrazafen
- CAS Number:63119-27-7
- Molecular formula:C18H17N3O2
- Molecular Weight:307.35
Yield:-
Reaction Conditions:
with n-butyllithium;sodium carbonate in tetrahydrofuran;water;
Steps:
1.C Preparation of 5,6-Bis(4-methoxyphenyl)-3-methyl-1,2,4-triazine
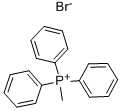
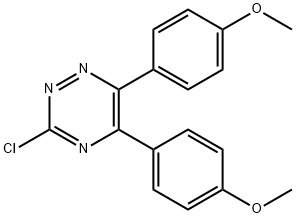
(C) 5,6-Bis(4-methoxyphenyl)-3-methyl-1,2,4-triazine. To a slurry of 11.7 g. (0.33 mole) of methyltriphenylphosphonium bromide in 150 ml. of dry tetrahydrofuran at -35° C. was added, over a 15-minute period, 20 ml. (0.033 mole) of n-butyl lithium. The reaction mixture was stirred for one hour. To the reaction mixture at -35° to -40° C. was added over a 10-minute period a solution of 5.7 g. (0.0165 mole) of 3-chloro-5,6-bis(4-methoxyphenyl)-1,2,4-triazine in 50 ml. of tetrahydrofuran. The reaction mixture was allowed to warm to ambient temperature and was stirred overnight. A solution of 1.05 g. (0.0165 mole) of sodium carbonate in 50 ml. of water was added dropwise to the reaction mixture which then was heated at reflux for 3 hours. The reaction mixture was cooled, poured over ice, and extracted with diethyl ether. The diethyl ether extract was washed with water, dried over anhydrous sodium sulfate, and concentrated. The concentrate was chromatographed over silica gel, with three fractions being collected. After evaporation of solvent, the third fraction solidified, m.p. about 109°-113° C. The solid was identified as 5,6-bis(4-methoxyphenyl)-3-methyl-1,2,4-triazine by nuclear magnetic resonance analysis, mass spectrographic analysis, and elemental micro-analysis. Analysis: C18 H17 N3 O2. Calc: C, 70.34; H, 5.58; N, 13.67; Found: C, 70.42; H, 5.66; N, 13.33.
References:
US4021553,1977,A