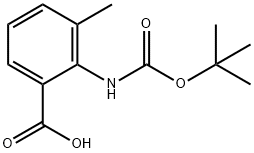
ANTHRANILIC ACID, N-BOC-3-METHYL synthesis
- Product Name:ANTHRANILIC ACID, N-BOC-3-METHYL
- CAS Number:669713-59-1
- Molecular formula:C13H17NO4
- Molecular Weight:251.28

24424-99-5
825 suppliers
$13.50/25G

4389-45-1
472 suppliers
$5.00/5g

669713-59-1
33 suppliers
$58.00/2g
Yield:-
Reaction Conditions:
with dmap;triethylamine in acetonitrile at 20; for 2 h;
Steps:
General experimental procedure
General procedure: To a solution of anthranilic acid (0.5g, 3.64mmol) in acetonitrile (10mL) were added TEA (0.73g, 7.28mmol), Boc anhydride (0.95g, 4.37mmol), and DMAP (0.044g, 0.36mmol). The mixture was stirred at rt for 2.0 h. CMPI (1.1g, 4.36mmol) was added in one lot to the reaction mixture. The reaction mixture was stirred at rt for 10 min. 1N HCl (20mL) was added to the reaction and the mixture was extracted two times with EtOAc. The combined organic layers were washed with brine, dried with anhydrous Na2SO4, filtered, and concentrated under reduced pressure. The resulting solid was crystallized in DCM and MeOH to afford 0.56g (95%) of 1H-benzo[d][1,3]oxazine-2,4-dione as an off white solid. 1H NMR (400MHz, DMSO) δ 7.14-7.16 (d, J=8Hz, 1H), 7.23-7.27 (t, J=16Hz,1H), 7.72-7.74 (t, J=8Hz, 1H), 7.90-7.92 (d, J=8Hz,1H), 11.72 (s, 1H). 13C NMR (100MHz, DMSO-d6) δ 110.6, 115.7, 123.9, 129.3, 137.3, 141.8, 147.5, 160.3. IR (thin film) 3461, 3173, 2937, 1984, 1753, 1604, 1486, 1358, 1258, 1138, 1009, 765, 673, 492. ES-MS (m/z): 161.8 (M+-H). MP (°C): 236°.
References:
Verma, Chhaya;Sharma, Somesh;Pathak, Arunendra [Tetrahedron Letters,2013,vol. 54,# 50,p. 6897 - 6899] Location in patent:supporting information