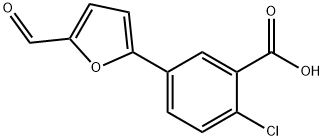
ASISCHEM R44416 synthesis
- Product Name:ASISCHEM R44416
- CAS Number:355142-36-8
- Molecular formula:C12H7ClO4
- Molecular Weight:250.63
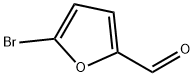
1899-24-7
266 suppliers
$13.43/500mgs:
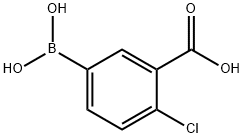
913835-32-2
106 suppliers
$6.00/100mg

355142-36-8
18 suppliers
$121.00/250mg
Yield:355142-36-8 87%
Reaction Conditions:
with tetrakis(triphenylphosphine) palladium(0);potassium carbonate in ethanol;toluene at 90; for 15 h;Inert atmosphere;
Steps:
1.3 Step 3: Synthesis of 2-Chloro-5-(5-formylfuran-2-yl)benzoic acid (7):
General procedure: A solution of K2C03 (2.37 gm, 3 equiv.) in water (10 mL) was added to a mixture of 4-chloro-3-carboxyphenylboronic acid (1.37 gm, 1.2 equiv.) and 5-bromo-2-furaldehyde (1 gm, 1 equiv.) in toluene/ethanol (60 mL). The mixture was degassed with argon for 5 minute and then Pd(PPh3)4 (330 mg, 0.05 equiv.) was added. The reaction mixture was stirred at 90°C for 15 h. The reaction mixture was cooled to room temperature, filtered through Celite and was with water (2 x 10 mL). The pH of solution was adjusted to 1-2 by addition of 6N HC1 solution. The precipitated reaction mixture was extracted with dichloromethane (3 x 100 mL); the combined organic fractions were washed with brine, dried over anhydrous Na2504, and concentrated under reduced pressure. The crude product was triturated with 20-30% EtOAc in hexanes (2 times), solid was filtered to afford 2-chloro-5-(5-formylfuran-2- yl)benzoic acid 7 (1.24 gm, 87% yield) as an off-white solid. TLC: 60% EtOAc in hexanes, Rf = 0.40 visualized with UV and KMnO4 solution.‘H NMR (300 MHz, DMSO): (5 13.74 (brs, 1H, COON), 9.63 (s, 1H, CHO), 8.23 (d, 1H, J = 2.22 Hz), 8.01 (dd, 1H, J = 2.28 and 8.43 Hz), 7.70 (d, 1H, J = 8.34 Hz), 7.67 (d, 1H, J = 2.85 Hz), 7.45 (d, 1H, J = 3.75 Hz); ‘3C NMR (75 MHz, DMSO): (5 178.64, 166.63, 156.44, 152.47, 132.93, 132.78, 132.13, 129.00, 128.10, 127.23, 110.56.
References:
WO2017/205503,2017,A1 Location in patent:Page/Page column 40
752231-43-9
6 suppliers
$46.00/1g

355142-36-8
18 suppliers
$121.00/250mg
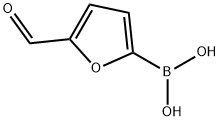
27329-70-0
459 suppliers
$6.00/1g
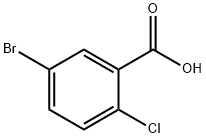
21739-92-4
638 suppliers
$8.00/10g

355142-36-8
18 suppliers
$121.00/250mg
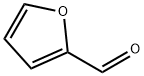
98-01-1
478 suppliers
$22.00/25g

89-54-3
251 suppliers
$13.00/5g

355142-36-8
18 suppliers
$121.00/250mg

89-54-3
251 suppliers
$13.00/5g

355142-36-8
18 suppliers
$121.00/250mg