
ATRAZINE-DESETHYL synthesis
- Product Name:ATRAZINE-DESETHYL
- CAS Number:6190-65-4
- Molecular formula:C6H10ClN5
- Molecular Weight:187.63
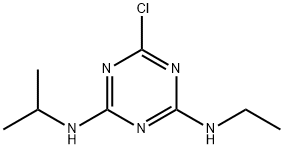
1912-24-9
355 suppliers
$28.00/100mg
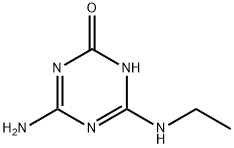
7313-54-4
21 suppliers
$45.00/5mg
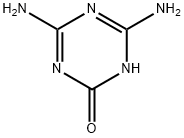
645-92-1
112 suppliers
$46.60/1g

6190-65-4
68 suppliers
$60.00/250mg
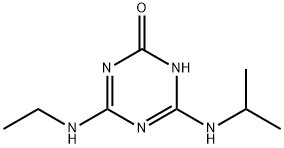
2163-68-0
48 suppliers
$113.00/250mg
Yield:-
Reaction Conditions:
with peroxymonosulfate at 24.84; pH=5.09;Kinetics;Sonication;pH-value;Temperature;Concentration;Reagent/catalyst;
Steps:
2.4. Experimental procedure
Batch experiments were carried out to investigate the performance of applied UV/CoFe2O4MWCNT/PMS and US/CoFe2O4MWCNT/PMS systems in degradation of ATZ, and to determine the influence of experimental conditions on the system efficiency. For degradation experiments, 100mL of ATZ solution was used. The effect of initial pH was investigated in the pH range of 3.0-11.0. The solution pH was adjusted by addition of small amounts of sulfuric acid (0.1N) and sodium hydroxide (0.1M) solutions. The effect of catalyst concentrations in the range of 0.05-1g/L was investigated at optimum pH. At the next steps, the effect of different concentrations of PMS (0.25-5mM) and different concentrations of ATZ (20-100mg/L) on the atrazine degradation efficiency was studied. In the optimum conditions obtained from the previous steps, the effect of different temperatures (283, 298, and 313K) on the removal efficiency was also determined. In UV/CoFe2O4MWCNT/PMS process, experiments were carried out in a 150mL quartz reactor under UV irradiations (UVA: 365nm, UVC: 254nm). In US/CoFe2O4MWCNT/PMS process, experiments were performed in a 1000mL glass container under US irradiation (US power: 100-400 W). The samples were collected at different time intervals and then, were filtrated by a 0.2μm PTFE syringe filter for atrazine analysis. After each sampling, methanol, and NaNO2 were used as quenching agents for radicals' inactivation and terminating the reaction. The mineralization was evaluated in terms of chemical oxygen demand (COD) and total organic carbon (TOC). The reusability of the nanocomposite was carried out by repetitive experiments at optimum conditions for five consecutive runs. For investigation of atrazine removal kinetic, the atrazine degradation efficiency was measured at different reaction times (2-60min) under optimum conditions. For determine the degradation rate constant of ATZ as a function of reaction operating parameters, the pseudo-first-order kinetic model was fitted based on Eq. (7). (7) LnCCo=-kobst LnCCo=-kobst where C and Co are the final and initial concentrations of ATZ (mg/L), respectively. kobs is the reaction rate constant (min-1) and t is the time of reaction (min) [27,36].
References:
Dehvari, Mahboobeh;Babaei, Ali Akbar;Esmaeili, Shirin [Journal of Photochemistry and Photobiology A: Chemistry,2023,vol. 437,art. no. 114452]
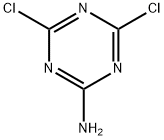
933-20-0
139 suppliers
$12.00/250mg
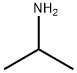
75-31-0
414 suppliers
$14.00/25mL

6190-65-4
68 suppliers
$60.00/250mg

1912-24-9
355 suppliers
$28.00/100mg

6190-65-4
68 suppliers
$60.00/250mg

2163-68-0
48 suppliers
$113.00/250mg

1007-28-9
62 suppliers
$50.00/25mg

1912-24-9
355 suppliers
$28.00/100mg
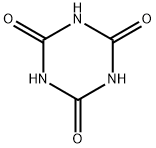
108-80-5
476 suppliers
$6.00/25g

6190-65-4
68 suppliers
$60.00/250mg

2163-68-0
48 suppliers
$113.00/250mg

1007-28-9
62 suppliers
$50.00/25mg

1912-24-9
355 suppliers
$28.00/100mg

3397-62-4
148 suppliers
$22.00/1g

6190-65-4
68 suppliers
$60.00/250mg

1007-28-9
62 suppliers
$50.00/25mg