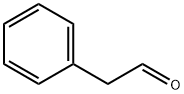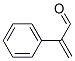
Atropaldehyde synthesis
- Product Name:Atropaldehyde
- CAS Number:4432-63-7
- Molecular formula:C9H8O
- Molecular Weight:132.16
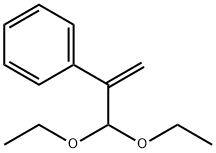
80234-04-4
4 suppliers
inquiry

4432-63-7
3 suppliers
inquiry
Yield:4432-63-7 74%
Reaction Conditions:
with formic acid in water at 0 - 20; for 0.666667 h;
Steps:
5. Synthesis of 4 via the hydrolysis of 3a[
A 10 mL tube containing a stirring bar was charged with formic acid (60 μL) and water (40μL). After cooling to 0 oC, the tube was dropped with atropaldehyde diethyl acetal 3a (47.2 mg,0.2 mmol), and stirred for 40 min under room temperature. Then, the mixture was extracted withethyl acetate (3 × 4 mL). The organic layers washed by brine were combined and dried over withMgSO4, concentrated under reduced pressure. Column chromatography was performed on silicagel to afford the product 2-phenylacrylaldehyde[9] (4) (24.3 mg, 74% yield) as a colorless oil. 1HNMR (500 MHz, CDCl3) δ: 9.83 (s, 1H), 7.48-7.46 (m, 2H), 7.42-7.37 (m, 3H), 6.64 (s, 1H), 6.20(s, 1H).
References:
Zhang, Li [Synlett,2021,vol. 32,# 7,p. 723 - 727] Location in patent:supporting information
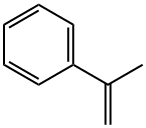
98-83-9
293 suppliers
$10.00/25mL

4432-63-7
3 suppliers
inquiry
201230-82-2
1 suppliers
inquiry
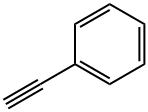
536-74-3
295 suppliers
$10.00/1g
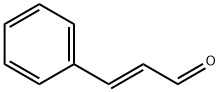
14371-10-9
317 suppliers
$6.00/25g

4432-63-7
3 suppliers
inquiry