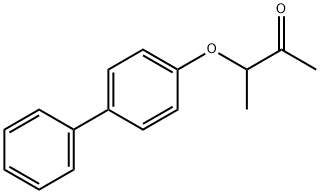
AURORA 17336 synthesis
- Product Name:AURORA 17336
- CAS Number:28089-74-9
- Molecular formula:C16H16O2
- Molecular Weight:240.3
Yield:28089-74-9 100%
Reaction Conditions:
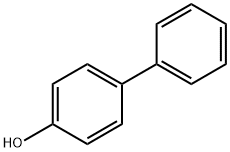
Stage #1: 4-Phenylphenolwith 18-crown-6 ether;potassium carbonate in butanone at 20; for 0.5 h;Inert atmosphere;
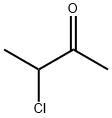
Stage #2: 3-chloro-2-butanone in butanone at 20; for 23 h;Inert atmosphere;
Steps:
3.1.5. 3-(4-Phenylphenoxy)butan-2-one (57)
Under a nitrogen atmosphere, a mixture of 4-phenylphenol(1.35 g, 7.93 mmol), 18-crown-6 ether (50 mg, 0.19 mmol), K2CO3(1.10 g, 7.96 mmol) and dry butan-2-one (20 mL) was stirred atroom temperature for 30 min. Then 3-chlorobutan-2-one (530 mL,502 mg, 4.71 mmol) was added dropwise and stirring wascontinued at room temperature for 23 h. The reaction mixture wasdiluted with saturated aqueous NaCl solution and exhaustivelyextracted with ethyl acetate. The combined organic layers weredried over anhydrous Na2SO4 and concentrated. The residue waspurified by chromatography on silica gel (cyclohexane to cyclohexane/ethyl acetate, 9:1) to yield 57 as a solid (1.13 g, 100%).C16H16O2 (240.3); mp 38-39 °C; 1H NMR (400 MHz, DMSO-d6):δ (ppm) 1.45 (d, J 6.8 Hz, 3H), 2.19 (s, 3H), 4.95 (q, J 6.8 Hz, 1H),6.92-7.00 (m, 2H), 7.26-7.36 (m, 1H), 7.38-7.49 (m, 2H), 7.55-7.65(m, 4H); HRMS (APCI, direct probe) m/z [M+H+] calculated:241.1229, found: 241.1243.
References:
Garzinsky, David;Zahov, Stefan;Ekodo Voundi, Merlin;Hanekamp, Walburga;Lehr, Matthias [European Journal of Medicinal Chemistry,2018,vol. 160,p. 183 - 192]
92-69-3
414 suppliers
$6.00/25g
814-75-5
100 suppliers
$21.21/250mgs:

28089-74-9
10 suppliers
$23.00/1g