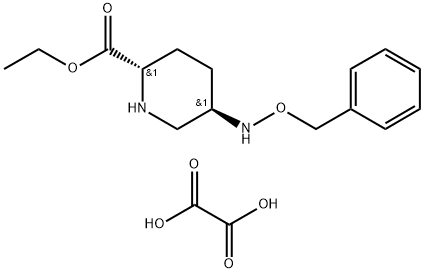
Avibactam INT 1 synthesis
- Product Name:Avibactam INT 1
- CAS Number:1416134-48-9
- Molecular formula:C17H24N2O7
- Molecular Weight:368.39

64-17-5
732 suppliers
$10.00/50g
![Benzyl (2S,5R)-5-[(benzyloxy)amino]piperidine-2-carboxylate ethanedioate](/CAS/20180703/GIF/1171080-45-7.gif)
1171080-45-7
104 suppliers
inquiry
141-52-6
476 suppliers
$10.00/5g

144-62-7
775 suppliers
$5.00/5g

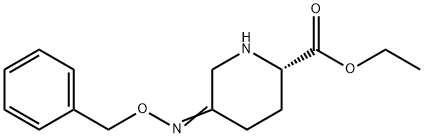
1416134-48-9
235 suppliers
inquiry
Yield:1416134-48-9 94%
Reaction Conditions:
Stage #1:ethanol;(2S,5R)-5-benzyloxyaminopiperidin-2-carboxylic acid benzyl ester oxalic acid salt;sodium ethanolate at 0; for 1 h;
Stage #2:oxalic acid in ethyl acetate;acetone at 35;
Steps:
1.1f
Example 1fEthyl (2S,5R)-5-[(benzyloxy)amino]piperidine-2-carboxylate ethanedioate (1:1) was prepared as described below.A slurry of benzyl (2S,5R)-5-[(benzyloxy)amino]piperidine-2-carboxylate ethanedioate (1:1) (100 g, 232 mmol) in ethanol (2000 ml) was cooled to 0° C. A solution of sodium ethoxide in ethanol (216 ml, 580 mmol, 21 wt % solution) was added slowly and the mixture was stirred for 1 h at 0° C. Acetic acid (13.3 ml, 232 mmol) was added and the mixture was concentrated under vacuum below 35° C. to a final volume of 300 ml. Ethyl acetate (700 ml) was added and the mixture was concentrated to 300 ml. This procedure was repeated twice. Water (1800 ml) was added to the mixture followed by aqueous ammonia (variable) until the pH of the aqueous layer was 7.5 to 8. The layers were separated and the aqueous layer was extracted with ethyl acetate (2×300 ml). The combined organic layers were washed with water (500 ml) and concentrated to a final volume of 300 ml. The solution was filtered and diluted with ethyl acetate (700 ml) and warmed to 35° C. A solution of oxalic acid dihydrate (30 g, 237 mmol) in acetone (200 ml) was added and the mixture was cooled to room temperature. The solids were isolated by filtration, washed with ethyl acetate and dried under vacuum at 35° C. to obtain ethyl (2S,5R)-5-[(benzyloxy)amino]piperidine-2-carboxylate ethanedioate (1:1) as a white solid (80.7 g, 94%).
References:
FOREST LABORATORIES HOLDINGS LTD. US2012/323010, 2012, A1 Location in patent:Page/Page column 17
1416134-60-5
44 suppliers
inquiry

144-62-7
775 suppliers
$5.00/5g

1416134-48-9
235 suppliers
inquiry
113400-36-5
170 suppliers
$6.00/10g

1416134-48-9
235 suppliers
inquiry
![2-Piperidinecarboxylic acid, 5-[(phenylmethoxy)imino]-, phenylmethyl ester, (2S)-](/CAS/20180703/GIF/1133931-74-4.gif)
1133931-74-4
7 suppliers
inquiry

1416134-48-9
235 suppliers
inquiry
![L-Norleucine, 6-chloro-N-[(1,1-dimethylethoxy)carbonyl]-5-[(phenylmethoxy)imino]-, phenylmethyl ester](/CAS/20180703/GIF/1133931-73-3.gif)
1133931-73-3
11 suppliers
inquiry

1416134-48-9
235 suppliers
inquiry