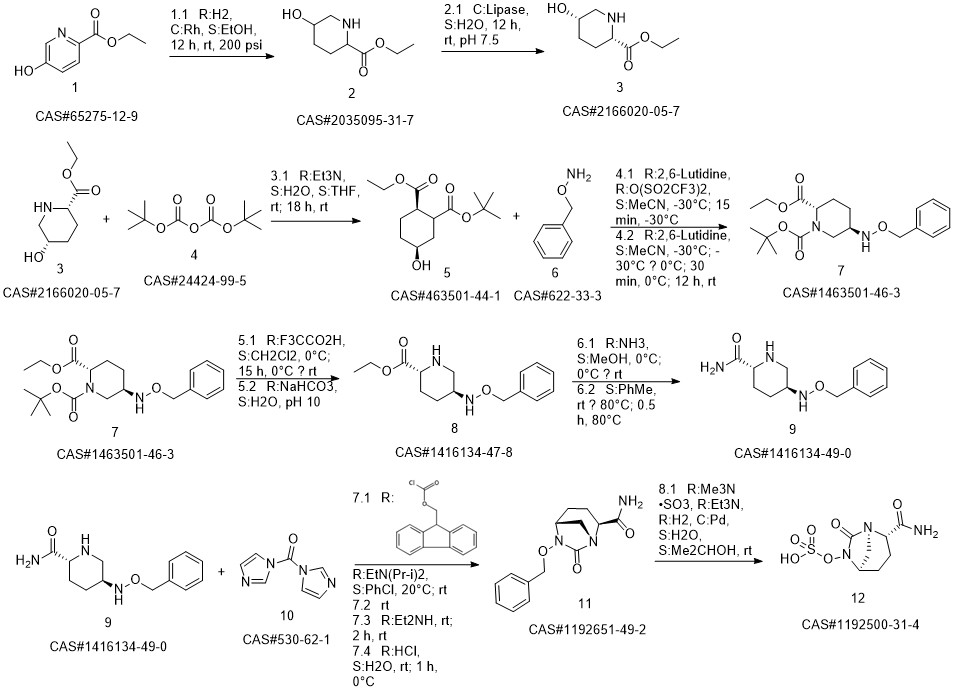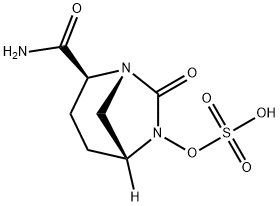
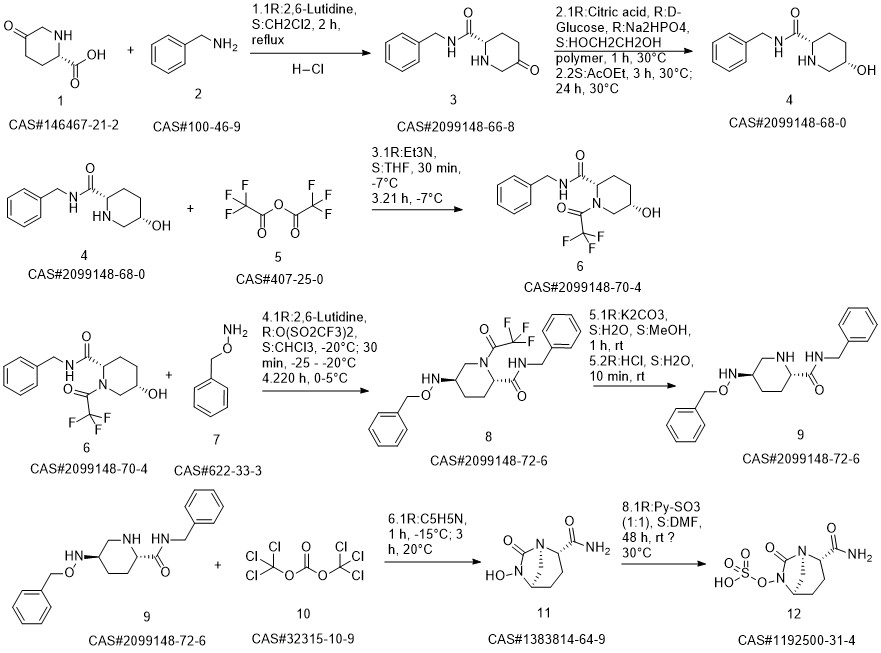
AvibactaM synthesis
- Product Name:AvibactaM
- CAS Number:1192500-31-4
- Molecular formula:C7H11N3O6S
- Molecular Weight:265.24
Reference: Liu, Chengxue; Tang, Feng; Zhang, Liming. Method for synthesizing β-lactamase inhibitor Avibactam. CN 106699756. (Assignee Zibo Xinquan Pharmaceutical Technology Service Co., Ltd., Peop. Rep. China)
![(2S,5R)-5-[(benzyloxy)amino]piperidine-2-carboxamide](/CAS/20180629/GIF/1416134-49-0.gif)
1416134-49-0
146 suppliers
inquiry

1192500-31-4
93 suppliers
inquiry
Yield:-
Steps:
Multi-step reaction with 2 steps
1: N-ethyl-N,N-diisopropylamine; (fluorenylmethoxy)carbonyl chloride / chlorobenzene / 30 °C
2: hydrogen / 5% Pd-C / dichloromethane; N,N-dimethyl-formamide / 2250.23 Torr
References:
FOREST LABORATORIES HOLDINGS LTD. US2012/323010, 2012, A1
![(2S,5R)-6-(benzyloxy)-7-oxo-1,6-diazabicyclo[3.2.1]octane-2-carboxamide](/CAS/20180629/GIF/1192651-49-2.gif)
1192651-49-2
114 suppliers
inquiry

1192500-31-4
93 suppliers
inquiry
![2-Piperidinecarboxylic acid, 5-[(phenylmethoxy)imino]-, phenylmethyl ester, (2S)-](/CAS/20180703/GIF/1133931-74-4.gif)
1133931-74-4
7 suppliers
inquiry

1192500-31-4
93 suppliers
inquiry
![L-Norleucine, 6-chloro-N-[(1,1-dimethylethoxy)carbonyl]-5-[(phenylmethoxy)imino]-, phenylmethyl ester](/CAS/20180703/GIF/1133931-73-3.gif)
1133931-73-3
12 suppliers
inquiry

1192500-31-4
93 suppliers
inquiry
![Benzyl (2S,5R)-5-[(benzyloxy)amino]piperidine-2-carboxylate ethanedioate](/CAS/20180703/GIF/1171080-45-7.gif)
1171080-45-7
96 suppliers
inquiry

1192500-31-4
93 suppliers
inquiry