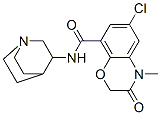
azasetron synthesis
- Product Name:azasetron
- CAS Number:123040-95-9
- Molecular formula:C17H20ClN3O3
- Molecular Weight:349.81
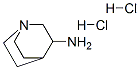
6530-09-2
154 suppliers
$6.00/250mg
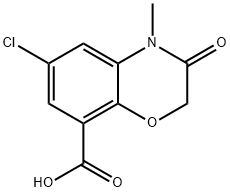
123040-79-9
94 suppliers
$19.00/250mg

123040-95-9
6 suppliers
inquiry
Yield:123040-95-9 86.3%
Reaction Conditions:
with O-(benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium tetrafluoroborate;triethylamine in dichloromethane at 0 - 10;
Steps:
2 Preparation of Compound IV
Into a 250 ml three-necked flask, 16.6 g of intermediate III, 15.1 g of 3-aminoquinuclidine dihydrochloride and 24.3 g of TBTU were added, followed by the addition of 150 ml of methylene chloride. The mixture was sufficiently mixed to lower the temperature of the system to 0 At 10 ° C, 23.0 g of triethylamine was added dropwise. After 20 min, the reaction was completed at 0 to 10 ° C for 1.5 h. TLC was followed until the reaction was complete. Washed with 75 ml of saturated aqueous sodium chloride twice, 75 ml of purified water twice, and then methylene chloride was distilled off under reduced pressure, and further recrystallized from ethyl acetate to give an intermediate IV of 20.8 g in a yield of 86.3% and a purity of 99.2%.
References:
CN103351386,2016,B Location in patent:Paragraph 0015; 0030; 0031
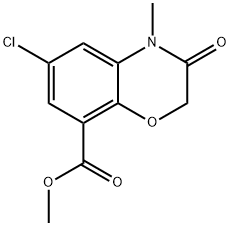
141761-83-3
79 suppliers
$102.00/1g

123040-95-9
6 suppliers
inquiry
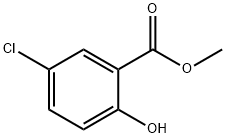
4068-78-4
194 suppliers
$12.00/5g

123040-95-9
6 suppliers
inquiry
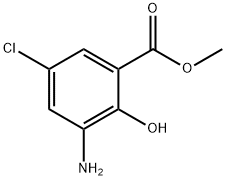
5043-81-2
125 suppliers
$31.00/250mg

123040-95-9
6 suppliers
inquiry
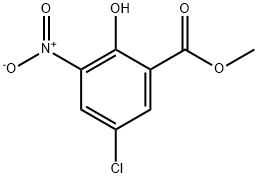
5043-79-8
63 suppliers
$35.00/1g

123040-95-9
6 suppliers
inquiry