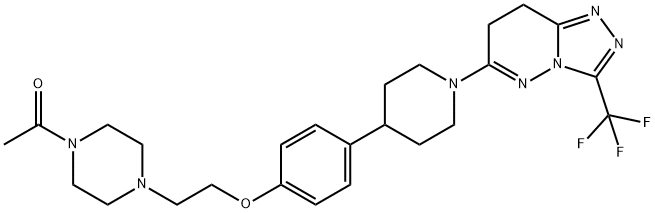
AZD3514 synthesis
- Product Name:AZD3514
- CAS Number:1240299-33-5
- Molecular formula:C25H32F3N7O2
- Molecular Weight:519.56
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

1240299-33-5
69 suppliers
inquiry
Yield: 88%
Reaction Conditions:
with hydrogen;palladium 10% on activated carbon in methanol at 50; under 3750.38 Torr; for 10 h;Product distribution / selectivity;Autoclave;
Steps:
5.5
Example 5.5; Alternative route for the preparation of 6-(4-{4-[2-(4-acetylpiperazin-l- vDethoxyl phenyllpiperidin- l-vD-3-f trifluoromethyl)-7.,8-(iihv(iro [ 1 ,2,41 triazolo [4,3- blpyridazine Form A; Methanol (375.0 mL) was added to 6-[4-[4-[2-(4-acetylpiperazin-l- yl)ethoxy]phenyl]piperidin-l-yl]-3-(trifluoromethyl)[ 1,2,4] triazolo[4,3-b]pyridazine (25.0 g, 48 m mol) in a 2.0 L autoclave reactor and to this was added 10% Pd/C (12.5 g, 50% w/w) paste at 22-25°C under nitrogen gas atmosphere. The reaction was performed under hydrogen pressure (5.0 bar) at 500C temperature for 10.0 h. The reaction mass was cooled to room temperature and the catalyst removed by filtration. Filtered cake was washed with methanol. The solvent was evaporated and the residue was azeotropically distilled by ethylacetate (2 x 125.0 mL) at 400C under reduced pressure to 3.0 rel vol (75.0 mL). Drop wise addition of tert-butylmethylether (MTBE, 375.0 mL) to the reaction mass resulted in solid material, which was collected by filtration and washed with MTBE (50.0 mL). The material was dried under reduced pressure with nitrogen gas bleed at 500C to afford the desired product 6-(4-{4-[2-(4-acetylpiperazin-l-yl)ethoxy]phenyl}piperidin-l-yl)-3- (trifluoromethyl)-7,8-dihydro[l,2,4]triazolo [4,3-b]pyridazine (22.3 g, 88%) as a white color free flowing solid. The isolated material was confirmed by XRPD as Form A. IH NMR (400.13 MHz, CDC13): δ 1.62 (2H, m), 1.88 (2H, m), 2.02 (3H, s), 2.49 (4H, m), 2.65 - 2.78 (5H, m), 2.94 (2H, m), 3.15 (2H, t), 3.42 (2H, m), 3.57 (2H, m), 4.03 (2H, t), 4.24 (2H, m), 6.80 (2H, d), 7.06 (2H, d); m/z = 520 [M+H]+.
References:
ASTRAZENECA AB;ASTRAZENECA UK LIMITED;BRADBURY, Robert, Hugh;CARR, Gregory, Richard;RABOW, Alfred, Arthur;RAO KORUPOJU, Srinivasa;TUMMA, Harikrishna WO2010/92371, 2010, A1 Location in patent:Page/Page column 81-82
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

1240299-33-5
69 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
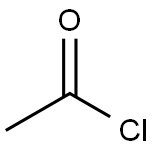
75-36-5
562 suppliers
$17.92/100G

1240299-33-5
69 suppliers
inquiry
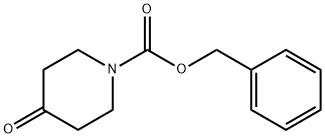
19099-93-5
318 suppliers
$5.00/1g

1240299-33-5
69 suppliers
inquiry

1166820-03-6
3 suppliers
inquiry

1240299-33-5
69 suppliers
inquiry