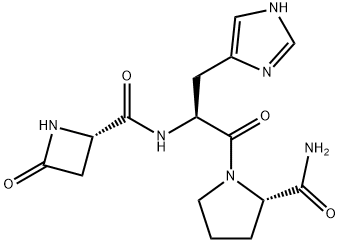
Azetirelin synthesis
- Product Name:Azetirelin
- CAS Number:95729-65-0
- Molecular formula:C15H20N6O4
- Molecular Weight:348.36
Yield:-
Reaction Conditions:
Stage #1: (S)-4-oxo-azetidine-2-carboxylic acidwith 2,3,4,5,6-pentafluorophenol;dicyclohexyl-carbodiimide in 1,4-dioxane;N,N-dimethyl-formamide at 0; for 3.5 h;
Stage #2: L-histidinyl-L-prolinamide in 1,4-dioxane;N,N-dimethyl-formamide at 0; for 2 h;
Steps:
7.13.2
Nα-(tert-butoxycarbonyl)-L-histidyl-L-prolinamide (4.0 g, 8.80 mmol) was dissolved in CH2Cl2 (30 mL) and the solution cooled to 0° C. Trifluoroacetic acid (25 mL) was added and the resulting solution stirred at 0° C. for 1 h. Concentration at RT under reduced pressure yielded a crude residue that was triturated with ether (40 mL) to afford the trifluoroacetate salt of L-histidyl-L-prolinamide as a white solid. The product was dried and used for the subsequent reaction as it was. It was dissolved in DMF (6 mL), and the solution was cooled to 0° C.; then Et3N (0.53 mL, 3.82 mmol, 1.05 equiv) was added dropwise. In a mixture of dioxane (20 mL) and DMF (7 mL) were dissolved at 0° C. (S)-4-OXO azetidine-2-carboxylic acid (1.02 g, 8.80 mmol, 1.0 equiv), DCC (2.01 g, 9.74 mmol, 1.1 equiv), and pentafluorophenol (1.38 g, 9.74 mmol, 1.1 equiv). The resulting mixture was stirred at 0° C. for 1.5 h then the above solution of the free amine was added and the mixture stirred an additional 2 h at 0° C. Insoluble matters were filtered off and the filtrate was concentrate under reduced pressure. The crude residue was purified by silica gel column chromatography (CHCl3/MeOH/NH3 40/10/1) to afford the title compound (2.66 g, 87%) as a white solid: 1H NMR (DMSO-d6) δ 1.74-1.90 (m, 3H), 1.92-2.10 (m, 1H), 2.65 (d, 1H, J=14.4 Hz), 2.83 (dd, 1H, J=5.7 and 14.7 Hz), 2.96 (dd, 1H, J=7.8 and 14.4 Hz), 3.09 (dd, 1H, J=5.1 and 14.1 Hz), 3.20-3.46 (m, 2H), 3.50-3.3.64 (m, 1H), 4.02 (dd, 1H, J=2.1 and 5.1 Hz), 4.18-4.25 (m, 1H), 4.66 (dd, 1H, J=7.2 and 13.5 Hz), 6.93 (s, 1H), 6.98 (br s, 1H), 7.57 (s, 1H), 8.03 (br s, 1H), 8.14 (br s, 1H), 8.38 (d, 1H, J=7.2 Hz).
References:
US7202279,2007,B1 Location in patent:Page/Page column 48

![(2S)-1-[(2S)-2-Amino-3-(3H-imidazol-4-yl)propanoyl]pyrrolidine-2-carboxamide](/CAS/GIF/33605-69-5.gif)