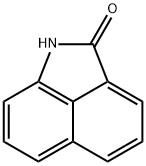
Benz[cd]indol-2(1H)-one synthesis
- Product Name:Benz[cd]indol-2(1H)-one
- CAS Number:130-00-7
- Molecular formula:C11H7NO
- Molecular Weight:169.18
Yield:130-00-7 80%
Reaction Conditions:
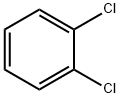
AlCl3 in (2S)-N-methyl-1-phenylpropan-2-amine hydrate;water
Steps:
40 EXAMPLE 40
EXAMPLE 40 292.6 g (2.2 mols) of anhydrous AlCl3 are introduced into 500 ml (650 g) of anhydrous o-dichlorobenzene, the suspension is warmed to 160° C., whilst stirring slowly, until the AlCl3 is dissolved and allowed to cool to 150° C., whilst stirring, and a solution of 169 g (1 mol) of 1-naphthyl isocyanate in 420 ml (540 g). of anhydrous o-dichlorobenzene is allowed to run in at 148°-153° C. in the course of 30 minutes. The mixture is then cooled to 80° C., the solution is discharged into a mixture of 3,000 g of ice water and 300 g of 37% strength HCl so that the temperature is kept below 30° C., the resulting mixture is stirred for 2 hours at temperatures of below 30° C. and the crystalline precipitate is filtered off, washed with 5 liters of water and dried at 90° C. in vacuo. Yield: 135.7 g of naphthostyril (= 80% of theory) Purity: 96% The same result is obtained if chlorobenzene or chlorotoluene is employed instead of o-dichlorobenzene and the reaction is carried out in a closed reaction vessel. If instead of 292.6 g (2.2 mols), 333 g (2.5 mols) of AlCl3 in 570 ml of o-dichlorobenzene are employed, 134 g (79% of theory) of naphthostyril are obtained with a purity of 96%.
References:
Bayer Aktiengesellschaft US4152330, 1979, A
![2-{[(4-methylphenyl)sulfonyl]oxy}-1H-benzo[de]isoquinoline-1,3(2H)-dione](/CAS/20180703/GIF/5551-72-4.gif)
5551-72-4
3 suppliers
inquiry
![Benz[cd]indol-2(1H)-one](/CAS/GIF/130-00-7.gif)
130-00-7
225 suppliers
$5.00/5g
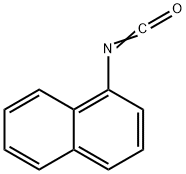
86-84-0
181 suppliers
$10.00/5g
![Benz[cd]indol-2(1H)-one](/CAS/GIF/130-00-7.gif)
130-00-7
225 suppliers
$5.00/5g

81-84-5
221 suppliers
$13.00/25g
![Benz[cd]indol-2(1H)-one](/CAS/GIF/130-00-7.gif)
130-00-7
225 suppliers
$5.00/5g
7797-81-1
115 suppliers
$11.00/1g
![Benz[cd]indol-2(1H)-one](/CAS/GIF/130-00-7.gif)
130-00-7
225 suppliers
$5.00/5g