
Benzaldehyde, 2,6-diethoxy- synthesis
- Product Name:Benzaldehyde, 2,6-diethoxy-
- CAS Number:107694-28-0
- Molecular formula:C11H14O3
- Molecular Weight:194.23
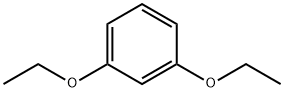
2049-73-2
80 suppliers
$26.67/10g

107694-28-0
4 suppliers
inquiry
Yield:107694-28-0 56%
Reaction Conditions:
with hydrogenchloride;n-butyllithium in hexane;water;N,N-dimethyl-formamide;
Steps:
1 EXAMPLE 1
A solution of 14.20 g of 1,3-diethoxybenzene in 250 ml of anhydrous ether, is added with 64 ml of a 1,6 M solution of n-butyllithium in n-hexane. The mixture is refluxed under nitrogen for 21 hours, then it is cooled with ice and treated with 9.9 ml of anhydrous N,N-dimethylformamide. The mixture is allowed to stand at room temperature overnight and then it is poured into a solution of 30 ml of concentrated hydrochloric acid in 200 ml of water, then stirred and extracted with 200 ml of ethyl ether, which volume is divided into two portions. The ether extracts are dried with anhydrous sodium sulfate and then they are evaporated to dryness. The residue gives after recrystallization from n-hexane the 2,6-diethoxybenzaldehyde with yield of 56%; melting point is 56°-58° C.
References:
US4888283,1989,A
108-46-3
749 suppliers
$10.00/10g

107694-28-0
4 suppliers
inquiry