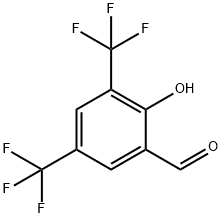
Benzaldehyde, 2-hydroxy-3,5-bis(trifluoromethyl)- synthesis
- Product Name:Benzaldehyde, 2-hydroxy-3,5-bis(trifluoromethyl)-
- CAS Number:1235783-06-8
- Molecular formula:C9H4F6O2
- Molecular Weight:258.12
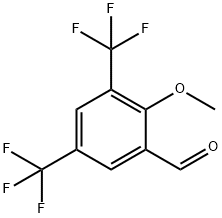
197015-73-9
4 suppliers
inquiry

1235783-06-8
2 suppliers
inquiry
Yield:1235783-06-8 70%
Reaction Conditions:
Stage #1: 2-methoxy-3,5-bis(trifluoromethyl)benzaldehydewith boron tribromide in dichloromethane at -78 - 25; for 20 h;
Stage #2: with water in dichloromethane at -40 - 20;
Steps:
1
Example 1Preparation of 2-hydroxy-3,5-bis-trifluoromethylbenzaldehyde 3,5-Bis(trifluoromethyl)anisaldehyde (prepared as in Sui and Macielag, Synth. Commun. 1997, 27, 3581-3590, which is expressly incorporated by reference herein; 2.0 grams (g), 7.7 millimoles (mmol)) was dissolved in dry dichloromethane (CH2Cl2; 15 milliliters (mL)), cooled to -78° C. and treated in portions with boron tribromide (BBr3, 1 M solution in CH2Cl2; 8.0 mL, 8.0 mmol). The mixture was stirred and allowed to warm to 25° C. After 20 hours (h), the mixture was cooled to -40° C., carefully treated with H2O (10 mL) and warmed to room temperature. The separated organic phase was washed with water (H2O; 10 mL), saturated (satd) sodium chloride (NaCl) solution (5 mL), dried over sodium sulfate (Na2SO4) and evaporated. The residue was purified by silica gel chromatography with a 0 to 20% gradient of ethyl acetate (EtOAc) in hexane to give the purified aldehyde (1.4 g, 70%) as an oil: 1H NMR (400 MHz, CDCl3) δ 12.05 (s, 1H), 10.02 (s, 1H), 8.07 (s, 2H). EIMS m/z 258.
References:
US2012/46170,2012,A1 Location in patent:Page/Page column 4