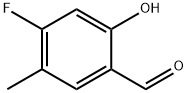
Benzaldehyde, 4-fluoro-2-hydroxy-5-methyl- (9CI) synthesis
- Product Name:Benzaldehyde, 4-fluoro-2-hydroxy-5-methyl- (9CI)
- CAS Number:504414-06-6
- Molecular formula:C8H7FO2
- Molecular Weight:154.14

50-00-0
848 suppliers
$10.00/25g
452-78-8
157 suppliers
$17.00/1g
920525-52-6
4 suppliers
inquiry

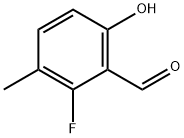
504414-06-6
23 suppliers
inquiry
Yield:504414-06-6 48% ,920525-52-6 11.5%
Reaction Conditions:
Stage #1: formaldehyd;3-fluoro-4-methyl phenolwith magnesium methanolate in methanol;toluene at 95; for 2 h;
Stage #2: with sulfuric acid in methanol;water;toluene at 35; for 2 h;
Steps:
11
Step 2. Preparation of 4-fluoro-2-hydroxy-5-methyl-benzaldehyde and 2-fluoro-6-hydroxy-3-methyl-benzaldehyde: The compound (2.26g, 17.920mmol) prepared in the step 1, was dissolved in magnesium methoxide in methanol solution (14.2ml, 10.762mmol, 8% wt in MeOH). Thereafter the reaction mixture was distilled until the methanol was left over about half point, thereto the reaction mixture was added toluene, and distilled when the reaction temperature was reached 95°C. p-Formaldehyde (1.66g, 155.18mmol) was slowly added for 1 hour, and then the reaction mixture was distilled for 1 hour, and cooled down room temperature. Then 10% sulfuric acid (20ml) was added, stirred at 350C for 2 hours. Ice water was added to quench the reaction and aqueous layer was extracted with toluene. Combined organic layer was dried over anhydrous magnesium sulfate and concentrated under reduced pressure. The residue was purified by column chromatography (n-hexane:ethyl acetate = 13:1) to give 1.25g (yield: 48.0%, colorless oil) of compound A and 0.3g (yield: 11.5%, colorless oil) of compound B. 1H NMR(400MHz, CDCl3, compound A): δ 11.15(s, IH), 9.98(s, IH), 7.36(d, J=8.4Hz, IH), 6.63(d, J=10.8Hz, IH), 2.23(s, 3H). 1H NMR(400MHz, CDCl3, compound B): δ 11.28(s, IH), 10.26(s, IH), 7.32(m,IH), 6.67(d, J=8.4Hz, IH), 2.10(s, 3H).
References:
WO2007/8037,2007,A1 Location in patent:Page/Page column 35