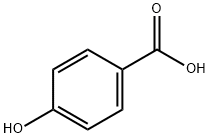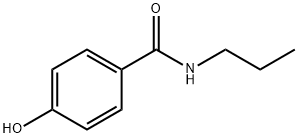
Benzamide, 4-hydroxy-N-propyl- synthesis
- Product Name:Benzamide, 4-hydroxy-N-propyl-
- CAS Number:27519-68-2
- Molecular formula:C10H13NO2
- Molecular Weight:179.22
Yield:27519-68-2 90%
Reaction Conditions:
with 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride in acetone;Heating;
Steps:
4.1.2. General procedure for synthesis of 4-hydroxybenzamides (5a-j)
General procedure: To a solution of 4-hydroxybenzoic acid (3, 690 mg, 5 mmol) inacetone (10 mL) at room temperature was added n-propylamine followed by EDCI (1.15 g, 6.0 mmol). After stirring the reaction mixturefor 12 h at 70 °C, the mixture was concentrated under vacuo, addedwater (10 mL), extracted with ethyl acetate (3×10 mL). The combinedorganic layers were washed with brine, dried over anhydrous sodiumsulfate, and concentrated under vacuo. The crude product was thenpurified by column chromatography using dichloromethane/acetone(20:1) to yield the pure 5a.Similar procedure as that described for 5a gave pure 5b-j.4.1.2.1. N-Propyl-4-hydroxybenzamide (5a). Light brown liquid; yield:90%; 1H NMR (600 MHz, DMSO-d6) δ 9.94 (s, 1H), 8.20 (s, 1H), 7.70 (d,J = 8.4 Hz, 2H), 6.78 (d, J = 8.4 Hz, 2H), 3.19-3.15 (m, 2H),1.52-1.48 (m, 2H), 0.87 (t, J = 7.2 Hz, 3H).
References:
Bo, Yong-Xin;Chen, Shi-Wu;Hao, Shu-Yi;Wang, Xing-Rong;Xiang, Rong;Xu, Yu [Bioorganic and medicinal chemistry,2020,vol. 28,# 5]
27519-67-1
2 suppliers
inquiry

27519-68-2
17 suppliers
inquiry
107-10-8
374 suppliers
$10.00/20mg
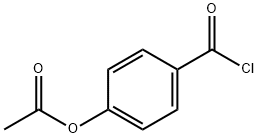
27914-73-4
20 suppliers
$63.61/250mgs:

27519-68-2
17 suppliers
inquiry
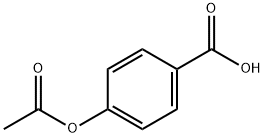
2345-34-8
194 suppliers
$13.00/5g

27519-68-2
17 suppliers
inquiry