
BenzaMide, N,2-dihydroxy-4-Methyl- synthesis
- Product Name:BenzaMide, N,2-dihydroxy-4-Methyl-
- CAS Number:158671-29-5
- Molecular formula:C8H9NO3
- Molecular Weight:167.16
4670-56-8
174 suppliers
$10.00/1g

158671-29-5
20 suppliers
inquiry
Yield:158671-29-5 99%
Reaction Conditions:
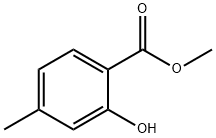
Stage #1: methyl 2-hydroxy-4-methylbenzoatewith sodium hydroxide in water;
Stage #2: methyl 2-hydroxy-4-methylbenzoate in 1,4-dioxane;water at 20; for 20 h;
Stage #3: with hydrogenchloride in 1,4-dioxane;water at 0; pH=5;
Steps:
1 2-N-Dihydroxy-4-methyl-benzamide:
A 250 ml round-bottom flask equipped with a stir bar was charged with hydroxylamine hydrochloride (3.5 g, 50.0 mmoles, 25.0 eq) followed by H2O (35 ml) and by an aqueous solution of NaOH (3.0M/H2O, 38 ml, 114 mmoles, 57.0 eq). In a separate flask, the crude 2-Hydroxy-4-methyl-benzoic acid methyl ester (332 mg, 2.0 mmoles, 1.0 eq) was dissolved in dioxane and added dropwise to the above solution. The reaction mixture was stirred at room temperature for 20 hrs, cooled to 0° C. on an ice bath and neutralized to pH=5 (pH paper strips) with conc. aqueous HCl (10.0M/H20). The reaction was allowed to warm to room temperature, EtOAc was added, the crude product was partitioned in a separatory funnel (3×EtOAc), the organic layer was dried with MgSO4 and filtered. Removal of excess solvent from the filtrate in vacuo provided 331 mg (99%) of the title compound as an off-white solid. (1H DMSO-d6, 400 MHz) δ 12.29 (s, 1H), 11.37 (s, 1H), 9.26 (s, 1H), 7.54 (d, J=8.0 Hz, 1H) 6.70 (s, 1H), 6.648 (d, J=8.1 Hz, 1H), 2.24 (s, 3H).
References:
US2005/143434,2005,A1 Location in patent:Page/Page column 9

50-85-1
330 suppliers
$10.00/5g

158671-29-5
20 suppliers
inquiry