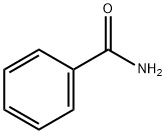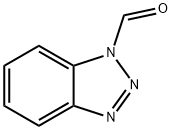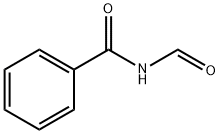
Benzamide, N-formyl- (7CI,8CI,9CI) synthesis
- Product Name:Benzamide, N-formyl- (7CI,8CI,9CI)
- CAS Number:4252-31-7
- Molecular formula:C8H7NO2
- Molecular Weight:149.15
Yield: 75%
Reaction Conditions:
Stage #1:benzamide with n-butyllithium in tetrahydrofuran;hexane at 0; for 0.0833333 h;Inert atmosphere;
Stage #2:N-formylbenzotriazole in tetrahydrofuran;hexane at 20; for 12 h;Inert atmosphere;
Steps:
N-Formylbenzamide, 12
A 0 °C solution of benzamide 11 (0.5 g, 4.13 mmol) in anhydrousTHF (40 mL) was treated with n-BuLi (3.1 mL, 4.96 mmol, 1.6 M solution in hexanes) andthe resulting solution was stirred at 0 °C for 5 mi n before being treated with Nformylbenzotriazole(0.91 g, 6.19 mmol). The reaction mixture was then allowed to warm upto room temperature and was stirred for a further 12 h. The reaction was diluted with tbutylmethylether (20 mL), and quenched with a saturated aq. NaHCO3 solution (40 mL).The layers were separated and the aqueous phase was extracted with diethyl ether (3 x 40mL). The combined organic layers dried over Na2SO4 and the solvent was removed undervacuum to yield a crude residue. Purification by flash column chromatography on silica gel(elution gradient 10 to 20% ethyl acetate in petroleum ether) afforded the desired N-formylimide 12 as a white solid in 75% yield (0.462 g, 3.1 mmol).1H NMR (500 MHz, CDCl3): δ 9.38 (1H, d, J = 9.8 Hz), 9.21 (1H, bs), 7.92 (2H, dd, J = 6.6,1.5 Hz), 7.67 (1H, tt, J = 7.5, 1.2 Hz), 7.55 (2H, td, J = 5.7, 1.5 Hz). 13C NMR (125 MHz,CDCl3): δ 166.8, 164.9, 134.1, 131.2, 129.2, 128.2. IR νmax (film) 3414, 1728, 1683, 1463,1364, 1252, 1208 cm-1. HRMS (CI+/ISO) calc. for C8H7NO2 [M]+: 149.0477. Found:149.0474
References:
Pasqua, Adele E.;Ferrari, Frank D.;Crawford, James J.;Marquez, Rodolfo [Tetrahedron Letters,2014,vol. 55,# 44,p. 6042 - 6043] Location in patent:supporting information
88070-48-8
2 suppliers
inquiry

4252-31-7
4 suppliers
inquiry
121876-96-8
0 suppliers
inquiry

4252-31-7
4 suppliers
inquiry
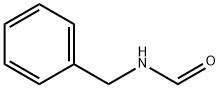
6343-54-0
97 suppliers
$7.00/1g

4252-31-7
4 suppliers
inquiry