
Benzenamine, 2,5-di-4-pyridinyl- synthesis
- Product Name:Benzenamine, 2,5-di-4-pyridinyl-
- CAS Number:1214384-80-1
- Molecular formula:C16H13N3
- Molecular Weight:247.29
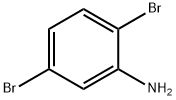
3638-73-1
204 suppliers
$10.00/1g
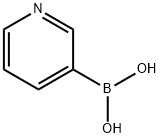
1692-25-7
488 suppliers
$5.00/1g

1214384-80-1
29 suppliers
inquiry
Yield:1214384-80-1 80.47%
Reaction Conditions:
with tris-(dibenzylideneacetone)dipalladium(0);tricyclohexylphosphine in 1,4-dioxane at 10; for 20 h;Inert atmosphere;
Steps:
1.1; 2.1; 3.1; 4.1; 5.1 (1) Synthesis of product I:
2,5-dibromoaniline (1.375 g, 5.5 mmol) was added to a 100 mL round bottom flask under N2 protection.3-pyridineboronic acid (2.44 g, 20 mmol),Tris(dibenzylideneacetone)dipalladium (364 mg, 0.4 mmol)And tricyclohexylphosphine (280 mg, 1 mmol),Additional 1,4-dioxane (40 ml, 41.32 g)And a potassium phosphate aqueous solution (20 ml) having a concentration of 0.25 to 0.28 g/ml,Reflow at 10 ° C for 20 h, TLC tracking.After completion of the reaction, the mixture was cooled to room temperature, and the catalyst was removed by filtration, and the mixture was extracted three times with ethyl acetate.The obtained solid was dissolved in chloroform, extracted three times with aqueous sodium carbonate, and the mixture was combined and dried over anhydrous magnesium sulfate.The crude product was dissolved in 80 mL of hot ethyl acetate, and the residue obtained by filtration of insoluble material was product 1 (1.03 g, yield: 80.47%).
References:
CN109438419,2019,A Location in patent:Paragraph 0027; 0030; 0033; 0036; 0039

3638-73-1
204 suppliers
$10.00/1g
1692-15-5
459 suppliers
$6.00/1g

1214384-80-1
29 suppliers
inquiry