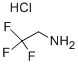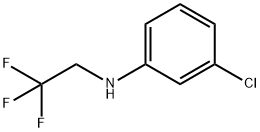
Benzenamine, 3-chloro-N-(2,2,2-trifluoroethyl)- synthesis
- Product Name:Benzenamine, 3-chloro-N-(2,2,2-trifluoroethyl)-
- CAS Number:351-45-1
- Molecular formula:C8H7ClF3N
- Molecular Weight:209.6
Yield:351-45-1 47%
Reaction Conditions:
Stage #1: 2,2,2-trifluroethylamine hydrochloridewith acetic acid;sodium nitrite in dichloromethane;water at 20; for 0.5 h;
Stage #2: 3-chloro-anilinewith C46H39FeN12O2 in dichloromethane;water; for 12.5 h;
Steps:
14 Synthesis of 3-chloro-N-(2,2,2-trifluoroethyl)aniline
2 mmol of trifluoroethylamine hydrochloride, 1 mL of water, 34 uL of acetic acid, and 1 mL of dichloromethane were placed in a reaction tube, and a rubber stopper was placed and fixed on a stirrer. Take 42 mg of sodium nitrite in a 1.5 mL sample tube and add 1 mL of water to the sample tube.Shake the sample tube to dissolve sodium nitrite.Dissolving the dissolved sodium nitrite solution into the reaction tube with a syringe,Stir for half an hour at room temperature.Dissolving the iron porphyrin of Formula 3 with 1 mL of dichloromethane (R2 = R3 = NH2, R1 = H, L = OAc) (catalytic amount,To 9/1000 of the molar amount of primary amine, 0.24 mmol of 3-chloroaniline was taken in the sample tube. After half an hour, add the mixed solution in the sample tube.The reaction tube was stirred and stirred for 12 hours. The reaction solution was cooled to room temperature, and some impurities were removed by filtration, and concentrated.The column chromatography was separated and purified to obtain the objective product, and the column chromatography eluent used was a mixed solvent of petroleum ether and acetone. 3-chloro-N-The structure of (2,2,2-trifluoroethyl)aniline is as follows:The compound was a pale yellow liquid with a yield of 47%
References:
CN108997144,2018,A Location in patent:Page/Page column 17

40410-54-6
9 suppliers
inquiry

351-45-1
5 suppliers
inquiry
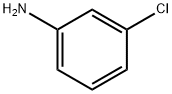
108-42-9
455 suppliers
$10.00/5g

351-45-1
5 suppliers
inquiry