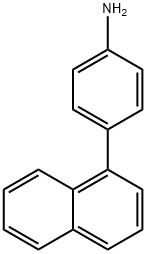
Benzenamine, 4-(1-naphthalenyl)- synthesis
- Product Name:Benzenamine, 4-(1-naphthalenyl)-
- CAS Number:125404-00-4
- Molecular formula:C16H13N
- Molecular Weight:219.28
Yield:125404-00-4 99%
Reaction Conditions:
with palladium diacetate;potassium carbonate;tris-(o-tolyl)phosphine in ethanol;water;toluene at 80; for 3 h;Inert atmosphere;
Steps:
1.2-1 Step 2-1: Synthesis of 1-(4-aminophenyl)naphthalene
Step 2-1:
Synthesis of 1-(4-aminophenyl)naphthalene
Synthesis Scheme (b-1) of Step 2-1 is shown.
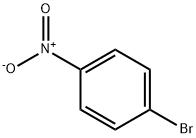
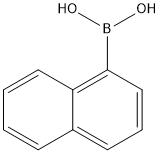
Into a 2-L three-neck flask were put 25 g (0.15 mol) of 4-bromoaniline, 25 g (0.15 mol) of 1-naphthaleneboronic acid, 450 mL of toluene, and 150 mL of ethanol.
While the pressure was reduced, this mixture was degassed by being stirred.
After the degassing, the atmosphere in a system was replaced with nitrogen.
Into the solution was added 220 mL (2.0 mol/L) of a potassium carbonate solution.
The obtained mixture was degassed by being stirred while the pressure was reduced, and then, the atmosphere in the system was replaced with nitrogen.
To this mixture were added 0.48 g (2.1 mmol) of palladium(II) acetate and 2.4 g (7.9 mmol) of tris(2-methylphenyl)phosphine, and the obtained mixture was stirred under a nitrogen stream at 80° C. for three hours.
After the stirring, this mixture was allowed to cool to room temperature, and an aqueous layer of this mixture was extracted three times with toluene.
The extracted solution and an organic layer were combined and washed twice with water and washed twice with saturated saline.
Into this mixture was added magnesium sulfate, and the mixture was dried for 18 hours.
The obtained mixture was subjected to natural filtration to remove magnesium sulfate, and the filtrate was concentrated to obtain an orange liquid.
This orange liquid was dissolved in toluene, and this solution was filtrated through Celite, alumina, and Florisil.
The obtained filtrate was concentrated to give 33 g of an objective orange liquid in a yield of 99%.
References:
Semiconductor Energy Laboratory Co., Ltd.;Shitagaki, Satoko;Hamada, Takao;Abe, Kanta;Seo, Satoshi US9419237, 2016, B2 Location in patent:Page/Page column 69; 70
13922-41-3
497 suppliers
$5.00/1g
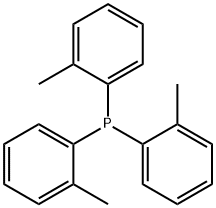
6163-58-2
443 suppliers
$5.00/1g
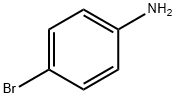
106-40-1
470 suppliers
$12.00/5g

125404-00-4
67 suppliers
inquiry