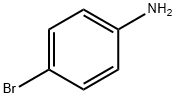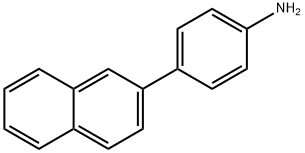
Benzenamine, 4-(2-naphthalenyl)- synthesis
- Product Name:Benzenamine, 4-(2-naphthalenyl)-
- CAS Number:209848-36-2
- Molecular formula:C16H13N
- Molecular Weight:219.28
Yield: 96%
Reaction Conditions:
with potassium phosphate;tetrakis(triphenylphosphine) palladium(0) in water;toluene at 100; for 2 h;Inert atmosphere;Sealed tube;
Steps:
9 2-(4-Aminophenyl)naphthalene was synthesized by the method shown below
Add to 100mL Schlenk tube with argon replacement equipped with stir bar4-bromoaniline (2.70g, 15.7mmol), 2-naphthylboronic acid (2.99g, 17.4mmol),Tetrakis (triphenylphosphine) palladium (543mg, 0.47mmol), toluene (30mL),Water (6mL) and tripotassium phosphate (3.81g, 31.4mmol), after sealing,Stir at 100°C for 2 hours. Then, cool the reaction vessel to near room temperature,Open the lid and add water (30 mL) to it. Move the contents to the separatory funnel,After the organic phase is separated from the aqueous phase, the aqueous phase is removed, and then the organic phase is washed with water.The organic phase is dried with sodium sulfate. Then, sodium sulfate is removed by filtration,The organic phase is concentrated. The concentrated mixture was purified by silica gel column chromatography (developing solvent: hexane/dichloromethane=1/3),Thereby obtaining the target 2-(4-aminophenyl)naphthalene(Yield 3.33g, yield 96%).
References:
Guandong Chemical Co., Ltd.;Xi Weitaiyi;Ji Guangdayou;Zuo Tenghuixing;Yan Jingxin;Xin Neicongchang CN111253264, 2020, A Location in patent:Paragraph 0264-0269
22082-99-1
199 suppliers
$8.00/250mg

209848-36-2
48 suppliers
inquiry
2765-24-4
0 suppliers
inquiry

209848-36-2
48 suppliers
inquiry
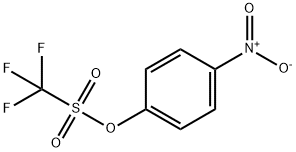
17763-80-3
77 suppliers
$20.69/1g

209848-36-2
48 suppliers
inquiry
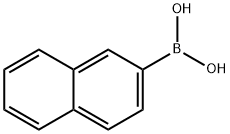
32316-92-0
474 suppliers
$6.00/1g

209848-36-2
48 suppliers
inquiry