
Benzenamine, 5-bromo-N-ethyl-2-methyl- synthesis
- Product Name:Benzenamine, 5-bromo-N-ethyl-2-methyl-
- CAS Number:1157928-89-6
- Molecular formula:C9H12BrN
- Molecular Weight:214.1
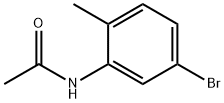
116436-10-3
24 suppliers
$75.00/100mg

1157928-89-6
2 suppliers
inquiry
Yield:1157928-89-6 69.3%
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran at 20; for 24 h;
Steps:
Roundbottom flask is charged with N-(5-bromo-2-methylphenyl)acetamide (Intermediate 137) ( 513.20 mg; 2.01 mmol; 1 .0 eq.) and anhydrous tetrahydrofuran (17.96 mL). RM is cooled down to 0 °C and lithium aluminium hydride 2.0 M solution in tetrahydrofuran ( 2.21 mL; 4.42 mmol; 2.2 eq.) is added in this temperature while RM is stirred. RM is allowed to warmed up to room temperature. RM is heated and stirred at reflux for 24 h. After this time RM is cooled to room temperature and water is added to RM. Then consecutively 5M aqueous solution of NaOH (5 mL) and water (10 mL). RM is stirred for 30 minutes and then extracted with EtOAc ( 3x 0 mL). Organic solvents are then collected, combined and washed with brine (2 times). Then organic solvents are dried over Na2SO4 overnight. After this organic solvent is evaporated to afford 5-bromo-N-ethyl-2-methylaniline ( 313.90 mg; yield 69.3 %; 95.0 % by UPLC) as a beige semisolid.
References:
WO2016/180536,2016,A1 Location in patent:Page/Page column 313
6358-77-6
152 suppliers
$6.00/250mg

1157928-89-6
2 suppliers
inquiry