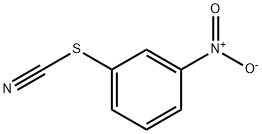
Benzene, 1-[(1-methylethyl)thio]-3-nitro- synthesis
- Product Name:Benzene, 1-[(1-methylethyl)thio]-3-nitro-
- CAS Number:70415-89-3
- Molecular formula:C9H11NO2S
- Molecular Weight:197.25
Yield:70415-89-3 77%
Reaction Conditions:
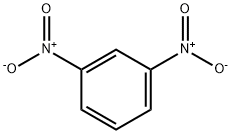
Stage #1: bis(3-nitrophenyl) disulfidewith potassium carbonate in N,N-dimethyl-formamide at 20; for 0.0333333 h;
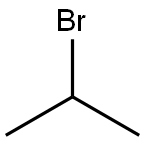
Stage #2: isopropyl bromidewith rongalite in water;N,N-dimethyl-formamide at 20; for 2 h;
Steps:
1-Nitro-3-(propan-2-ylsulfanyl)benzene (16)
A mixtureof 1,1-disulfanediylbis(3-nitrobenzene) 15 (3.00 g, 9.73 mmol)and K2CO3 (2.69 g, 19.5 mmol) in DMF (200 mL) was stirred at room temperature for 2 min. To this mixture were added 2-bromopropane (2.01 mL, 21.4 mmol), sodium hydroxymethanesulfinate(3.45 g, 29.2 mmol) and H2O (3 mL), and the reaction mixture was stirred at room temperature for 2 h. Water was added to this mixture, and the resulting slurry was extractedwith Et2O. The organic layer was washed with brine,dried over anhydrous MgSO4, and concentrated in vacuo togive 16 (2.95 g, 77%) as a yellow oil. 1H-NMR (400 MHz,CDCl3) δ: 1.32-1.40 (6H, m), 3.45-3.61 (1H, m), 7.41-7.52 (1H,m), 7.62-7.70 (1H, m), 8.01-8.09 (1H, m), 8.17-8.24 (1H, m).EI-MS m/z: 197 [M]+.
References:
Iikubo, Kazuhiko;Kondoh, Yutaka;Shimada, Itsuro;Matsuya, Takahiro;Mori, Kenichi;Ueno, Yoko;Okada, Minoru [Chemical and Pharmaceutical Bulletin,2018,vol. 66,# 3,p. 251 - 262]