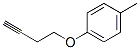
Benzene, 1-(3-butynyloxy)-4-methyl- (9CI) synthesis
- Product Name:Benzene, 1-(3-butynyloxy)-4-methyl- (9CI)
- CAS Number:391678-46-9
- Molecular formula:C11H12O
- Molecular Weight:160.2124
Yield:391678-46-9 40%
Reaction Conditions:
with triphenylphosphine;diethylazodicarboxylate in toluene at 0 - 70; for 24.5 h;Inert atmosphere;
Steps:
General procedure for synthesis of alkynes 2
General procedure: Phenol and its respective derivatives (containing -H -CH3 and -OCH3 groups at 4-position) were reacted with alcohols (but-3-yn-1-ol or pent-4-yn-2-ol) under Mitsunobu reaction conditions. Under Ar atmosphere, in three-necked flask phenol (50 mmol) was dissolved in dry toluene (50 ml) and corresponding alcohol (1 eq) and triphenylphosphine (1 eq) were added. Resulting solution was cooled to 0°C in ice-bath. DIAD (Diisopropyl azodicarboxylate, 1 eq) in dry toluene (20 ml) was added dropwise for 25 min and resulting mixture was stirred for additional 30 min, still cooling. Ise bath was removed and the mixture was stirred for next 16 h. Next it was stirred at 70°C for 8 h. After cooling to rt toluene was evaporated under reduced pressure. After dissolving in diethyl ether, side products were precipitated with hexanes and separated. Precipitation procedure was repeated and the residue was concentrated and purified with column chromatography using silica gel.
References:
Chmurski, Kazimierz;Stepniak, Pawel;Jurczak, Janusz [Synthesis,2015,vol. 47,# 13,art. no. SS-2015-T0083-PSP,p. 1838 - 1843] Location in patent:supporting information