
Benzene, 1-broMo-2-ethyl-4-(phenylMethoxy)- synthesis
- Product Name:Benzene, 1-broMo-2-ethyl-4-(phenylMethoxy)-
- CAS Number:668477-51-8
- Molecular formula:C15H15BrO
- Molecular Weight:291.18

668477-50-7
6 suppliers
inquiry

668477-51-8
8 suppliers
inquiry
Yield:668477-51-8 87%
Reaction Conditions:
with 1-bromopyrrolidine-2,5-dione in acetonitrile at 20; for 1.25 h;Cooling with ice;
Steps:
1.b (b)
4-(benzyloxy)-1-bromo-2-ethylbenzene (I-3)
To an ice cold stirred solution of 1-(benzyloxy)-3-ethylbenzene (I-2) (35.0 g, 164 mmol) in ACN (525 mL, 15 vol) was added N-bromosuccinimide (32.0 g 181 mmol) in portions over a period of 15 minutes.
The resulting reaction mixture was stirred for 1 hour at room temperature.
After completion of reaction (TLC monitoring), the resulting reaction mass was poured into ice cold water (1.50 L) followed by the extraction of compound with EtOAc (2*1 L).
The combined organics were washed with water and dried over sodium sulfate, filtered and evaporated under reduced pressure to obtain the crude product. n-Hexane (250 mL) was added to the crude material, resulting in a slurry, followed by filtration through a sintered funnel.
The mother liquor was evaporated under reduced pressure to obtain the desired product I-3 as a light yellow oily compound (42.0 g, 87%).
1H NMR (400 MHz, chloroform-d) δ 7.52-7.29 (m, 7H), 6.88 (s, 1H), 6.68 (d, J=6.0 Hz, 1H), 5.04 (s, 2H), 2.69 (q, J=7.6 Hz, 2H), 1.20 (t, J=7.5 Hz, 3H).
References:
US2020/71324,2020,A1 Location in patent:Paragraph 0090; 0092

620-17-7
191 suppliers
$32.89/10g

668477-51-8
8 suppliers
inquiry
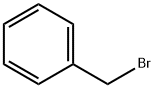
100-39-0
421 suppliers
$10.00/10g

668477-51-8
8 suppliers
inquiry